Lung diseases are very common conditions suffered by large number of people world-wide and are the causes of significant morbidity. Non-neoplastic lung diseases often present a diagnostic challenge to pathologists as well as clinicians. This domain incorporates interstitial lung disease, granulomatous conditions and infectious aetiologies to name a few [1]. The interstitial lung diseases are inflammatory and fibrosing conditions of the respiratory system largely affecting the alveolar parenchyma, alveolar spaces and rarely involves the bronchi and the bronchioles [2]. Pulmonary granulomatous lung disorders are miscellaneous conditions with heterogenous extent of pathologies, erratic clinical features and aftermaths [3]. Infectious respiratory diseases are more common than infections of other organs affecting all stages of life [4].
The non-neoplastic lung diseases are diagnosed based on primarily radiological evidence which is supported by lung function studies and haematological investigations. The wavering clinical and radiological characteristics of these diseases dictate the modus operandi for confirmation. Among the different affirmatory sampling methods, BAL is one of the most common means. It harvests the cellular and noncellular elements of the distal bronchioles and alveoli for cytology [5]. The prevalent cellular pattern in BAL analysis continually supports a diagnosis or helps narrow the differentials when it is studied in the milieu of patient history, systemic examination, and radiographic results [6]. TBLB is the most common and widely used technique of sampling lung tissue in pulmonary pathology since the evolution of the Flexible Bronchoscopy, in the 1970s [7]. TBLB increases the diagnostic yield and owing to the minimal risk associated with these procedures, airway endoscopy will remain crucial in the assessment of lung diseases [8]. The objectives of this study were to study the cell types in BAL fluid, classify the findings on BAL based on cellular patterns and find the concordance between BAL cellular pattern and TBLB in cases of non-neoplastic lung diseases.
Materials and Methods
A retrospective and descriptive study was undertaken in the Department of Pathology, Kempegowda Institute of Medical Sciences and Research Centre (KIMS&RC), Bengaluru, India. All the bronchoscopy procedures done from January to December in the year of 2019 were reviewed. The cytological and lung biopsy findings were compiled and statistical analysis was made in this study over a period of three months, from September to November 2020. Institutional Ethical Clearance-KIMS/IEC/A029/M/2020 was obtained for the study.
Inclusion criteria: The data of total of 40 patients who were clinically diagnosed with non-neoplastic lung disease and underwent combined BAL and TBLB during January to December 2019 at KIMS&RC were included in this study.
Exclusion criteria: Data of patients with severe coagulopathy, haemodynamics instability or those patients with neoplasms and inadequate biopsies were excluded from the study.
Study Procedure
The need for bronchoscopy was decided by the pulmonologist after reviewing the clinical and radiological discoveries. Informed consent was taken by pulmonologists before the bronchoscopy procedure, followed by asepsis. Anaesthetic medications, inhalation of 2% lignocaine through nebulisation and application of 2 mL lignocaine gel through the nostrils was administered before the procedure [9]. Trans-nasal flexible fibreoptic bronchoscopy was performed, using olympus bronchoscope. Around 100 mL of 0.9% saline was instilled which was followed by retrieval. The recovered volume of 10-20 mL was considered as optimum. Following the BAL, forceps were inserted through the bronchoscope channel into the Subsegmental bronchi by back-and-forth movement [10]. With the bronchoscope wedged into suitable bronchi, biopsies were taken from the required area. All the cases which were included had not received steroid or any other immunoregulatory therapy.
Differential cell counts were performed on the cell yield obtained by cytocentrifugation of the BAL followed by alcohol fixation. Minimum two slides were made in each case, one of which was stained with Papanicolaou stain and the other with Haematoxylin and Eosin (H&E) stain. A 400 cells on two separate cytology smears were observed and recorded to recognise the BAL nucleated cell profile [11]. The diagnostic criteria by the official American thoracic society clinical practice guidelines were followed [Table/Fig-1] to classify all the samples as lymphocytic, neutrophilic, eosinophilic and normal cellular pattern [12-14]. The differentials given for the distinct cellular pattern was noted. The predominant cellular pattern with its respective differential diagnosis is shown in the [Table/Fig-2]. A 10% buffered formalin was used for the collection of the TBLB specimens. The tissues were processed used customary procedures and were embedded in paraffin wax. Thin sections were made, mounted on glass slides and stained with standard H&E stain. The slides were now surveyed under a light microscope. The final diagnoses in all cases were made keeping the clinical presentation and radiology in mind. Evaluation of Bronchoalveolar cytology and TBLB histopathology was done independently by taking the latter as the gold standard. The diagnosis on TBLB was compared with the BAL differentials for each of the patients and was checked for concordance.
BAL cellular pattern in normal adults.
| Normal adults | BAL differential cell counts |
|---|
| Alveolar macrophages | >85% |
| Lymphocytes | 10-15% |
| Neutrophils | <3% |
| Eosinophils | <1% |
| Squamous epithelial cells/ciliated columnar epithelial cells | <5% |
BAL: Bronchoalveolar lavage
Disorders associated with increased percentage of specific BAL cell types [12].
| Lymphocytic cellular pattern | Eosinophilic cellular pattern | Neutrophilic cellular pattern |
|---|
| >15% lymphocytes | >1% eosinophils | >3% neutrophils |
| Sarcoidosis | Eosinophilic pneumonias | Collagen vascular diseases |
| Nonspecific interstitial pneumonia | Drug-induced pneumonitis | Idiopathic pulmonary fibrosis |
| Hypersensitivity pneumonitis | Bone marrow transplant | Aspiration pneumonia |
| Bronchiolitis obliterans organising pneumonia | Asthma, bronchitis | Infection: bacterial, fungal |
| Drug induced pneumonitis | Churg-strauss syndrome | Bronchitis |
| Collagen vascular disorders | Allergic bronchopulmonary aspergillosis | Asbestosis |
| Radiation pneumonitis | Bacterial, fungal, helminthic infections | Acute Respiratory Distress Syndrome (ARDS) |
| Lymphoproliferative disorders | Hodgkin’s disease | Diffuse Alveolar Damage (DAD) |
Statistical Analysis
The categorical data with respect to gender and cytomorphology of BAL fluid were expressed in proportions and the continuous data like age of patients, percentage of alveolar macrophages, neutrophils, lymphocytes and eosinophils were expressed in means and standard deviations. The validity of BAL compared to Histopathological Examination (HPE) was evaluated by calculating the sensitivity, specificity, positive predictive value, negative predictive value and overall diagnostic accuracy along with 95% confidence intervals. The extent of agreement/concordance in between the analytical patterns of BAL and HPE findings was estimated using kappa statistics. A kappa value (k) of less than 0.40 was considered to show poor agreement; that of 0.40-0.59 fair agreement; that of 0.60-0.74 good agreement; and that of 0.75-1.00 excellent agreement. The data analysis was conducted in SPSS version 18.0. A p-value of <0.05 was taken as statistically significant.
Results
This study included 40 patients investigated for non-neoplastic lung diseases based on the clinical and radiological findings. The median age of these cases was 51.5±15.75 years, including 24 (60%) males and 16 (40%) females (male:female=3:2). BAL was done in all the cases followed by TBLB. All the samples were categorised into four groups on the basis of predominant cellular pattern: Neutrophilic (n=13, 9 males and 4 females), Lymphocytic (n=16, 7 males and 9 females), Eosinophilic (n=5, all 5 of them were males) and normal BAL cytology (n=6, 3 males and 3 females). Among the 40 TBLB samples, the histopathological diagnosis was checked for concordance with the differential diagnosis on BAL. The highest diagnoses on TBLB are NSIP comprising 9 cases, followed by seven cases of UIP, six cases of BOOP, three cases of Bronchiolitis, two cases each of pulmonary tuberculosis and granulomatous inflammation, one case each of actinomycosis, sarcoidosis, lung abscess and mucor mycosis. Seven of the cases studied presented with normal BAL cytology. In patients diagnosed with NSIP, all of them had a lymphocytic cellular pattern, except one case which had normal BAL fluid cytology. Of the seven cases of UIP, six of the patients had a neutrophilic cellular pattern on BAL cytology whereas one of the cases presented with a lymphocytic cellular pattern. Four of the cases who presented with a lymphocytic cellular pattern and two of them who presented with neutrophilic cellular pattern were diagnosed as BOOP on histopathology. The highest discordance was found with neutrophilic cellular pattern (n=3), eosinophilic cellular pattern (n=2), normal BAL cytology (n=2) and least discordance was found with lymphocytic cellular pattern (n=1) [Table/Fig-3].
Discordant cases of BAL and TBLB.
| TBLB diagnosis | Corresponding cellular pattern | Cellular pattern by BAL fluid analysis |
|---|
| Bronchiolitis obliterans Organising pneumonia | Lymphocytic cellular pattern | Neutrophilic cellular pattern |
| Normal pathology | Normal BAL fluid cytology | Eosinophilic cellular pattern |
| Normal histology | Normal BAL fluid cytology | Neutrophilic cellular pattern |
| Usual interstitial pneumonia | Neutrophilic cellular pattern | Lymphocytic cellular pattern |
| Normal histology | Normal BAL fluid cytology | Neutrophilic cellular pattern |
| Bronchiolitis obliterans organising pneumonia | Lymphocytic cellular pattern | Normal BAL fluid cytology |
| Bronchitis | Lymphocytic cellular pattern | Eosinophilic cellular pattern |
| Nonspecific interstitial pneumonia | Lymphocytic cellular pattern | Normal BAL cytology |
TBLB: Transbronchial lung biopsy; BAL: Bronchoalveolar lavage
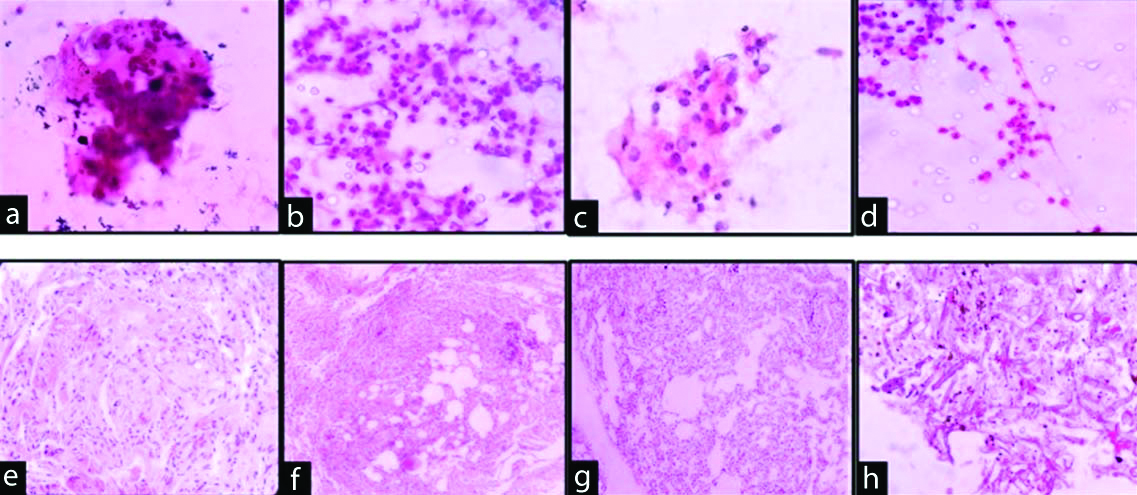
The cytological and the respective histopathological features of four cases are shown in the [Table/Fig-4a-d].
a) BAL showing multinucleated cell (H&E, 400X) and a: Granulomas of Sarcoidosis on HPE (H&E, 400X); b) Shows neutrophilic cellular pattern on BAL (H&E, 400X) and b: UIP on HPE (H&E, 100X); c) Shows lymphocytic cellular pattern on BAL (H&E, 400X) and c: HPE shows NSIP (H&E, 100X); d) Shows eosinophilic cellular pattern on BAL (H&E, 400X) and d: HPE shows Mucormycosis (H&E, ×400).
The measure of agreement/concordance among the two diagnostic methods was statistically significant with a Kappa value of 0.71 with 95% CI of 0.53-0.88 (p<0.05) as shown in the [Table/Fig-5]. The kappa value of 0.71 indicates a significantly good degree of agreement/ concordance between the two diagnostic methods.
Extent of agreement/concordance between BAL and TBLB in detecting non-neoplastic lung diseases.
| BAL | HPE | Overall Kappa (k) [95% CI] |
|---|
| Normal | Lymphocytic | Neutrophilic | Eosinophilic |
|---|
| Normal | 4 | 0 | 2 | 1 | 0.71 (0.53-0.88)p<0.05 |
| Lymphocytic | 2 | 15 | 1 | 1 |
| Neutrophilic | 0 | 1 | 10 | 0 |
| Eosinophilic | 0 | 0 | 0 | 3 |
HPE: Histopathological evaluation; bold p-value indicates statistical significance
The justification of BAL versus HPE in detecting non-neoplastic lung diseases was done with the data obtained in the present study. The evaluation parameters of BAL with respect to HPE indicated a sensitivity of 93.9%, specificity of 57.1%, positive predictive value of 91.1%, and negative predictive value of 66.6%; it had a positive likelihood ratio (LR) of 2.19 and a negative LR of 0.11. The overall accuracy of BAL test was 87.5%. Other parameters are shown in [Table/Fig-6,7].
Validation of BAL v/s HPE in detecting non-neoplastic lung diseases.
| Broncho-alveolar lavage findings | Histopathological examination findings | Total |
|---|
| Normal | Abnormal |
|---|
| Normal | 04 | 02 | 06 |
| Abnormal | 03 | 31 | 34 |
| Total | 07 | 33 | 40 |
Evaluation parameters of BAL in respect to TBLB.
| Evaluation parameters | Value | 95% CI |
|---|
| Sensitivity | 93.9% | 79.7-99.2 |
| Specificity | 57.1% | 18.4-90.1 |
| Positive predictive value | 91.1% | 81.3-96.0 |
| Negative predictive value | 66.6% | 31.1-89.8 |
| Positive likelihood ratio | 2.19 | 0.93-5.18 |
| Negative likelihood ratio | 0.11 | 0. 02-0.47 |
| Overall accuracy | 87.5% | 73.2-95.8 |
Discussion
Despite having capricious aetiologies, the clinical and radiological findings are analogous in most cases, making the diagnoses challenging. Differential cell counts of BAL fluid are concurrent investigations for these patients in centres which have Bronchoscopic facility [15]. Identification of cellular pattern in BAL cytology is beneficial both to the pulmonologist and pathologist as it tapers the list of differential diagnosis. Presence of dysplastic cells aids in the diagnosis of small and non small cell lung tumours. This study aimed to study the BAL cytology and tissue pathology on TBLB and uncover the concordance between them for non-neoplastic lung diseases. It was observed that patients with a lymphocytic cellular pattern on BAL cellular analysis were predominantly diagnosed as NSIP and BOOP. Meyer KC et al., found similar results in their study [13]. Welker L et al., stated that the likelihood of NSIP increased from 2.9% to 9.1% with higher lymphocyte numbers in BAL [16]. The cellular profile of excess BAL lymphocytes in the setting of peripheral alveolar infiltrates, in which infectious and malignant disease has been excluded by bronchoscopy, strongly suggests BOOP [17].
A lymphocyte differential count more than 25% suggests a diagnosis of granulomatous disease [18]. Two important granulomatous conditions in the present study are tuberculosis (n=2) and sarcoidosis (n=1). In paucibacillary tuberculosis, which has a high frequency in India, BAL cytology is widely used when confirmatory microbiology is lacking [19,20]. Greco S et al., studied 88 sarcoidosis and 76 tuberculosis patients and showed that there are significant differences in BAL lymphocyte percentage. In sarcoidosis, the median lymphocyte percentage was found to be 30% versus 14% in tuberculosis [21]. In patients with miliary tuberculosis, the lymphocyte count in BAL fluid were significantly increased [19,22].
The typical BAL cellular profile in UIP consists predominantly of increased percentage of neutrophils [23,24]. Neutrophil counts greater than 5% and sometimes up to 30% are seen in majority of patients. Of the seven UIP cases in this study the neutrophil count varied from (16-40%). Few studies state that the number of neutrophils may be directly proportional to the extent of disease seen on High-Resolution Computed Tomography (HRCT) and hence are associated with a worse diagnosis [25]. Significant BAL lymphocytosis suggests a diagnosis other than UIP [26].
Bodal VK et al., have reported that bronchoscopy is a useful diagnostic tool and BAL was the most effective material with which 50.66% of suspected tuberculosis cases turned out positive for Mycobacterium tuberculosis bacilli on staining with Auramine followed by fluorescent microscopy in cases which were earlier reported as negative for Acid-fast bacilli on Ziehl-Neelsen staining [27].
A study conducted by Jois DS et al., correlated the diagnostic concordance of BAL, brush cytology and biopsy imprint smears with histopathological features on TBLB [28]. The standardised method for the reporting of both histopathological and cytological specimens was used. They concluded that a combination of cytological techniques is more useful than a single technique for the evaluation of lung lesions. In their study, they found that for malignancies, imprint smears had more sensitivity and specificity when it was judged against other cytological tests. Cytology and biopsy had poor concordance and level of agreement when non-neoplastic diseases were considered in general. The concordance improved (42.5%) when imprint smears were added as an adjunct to BAL procedure. However, biopsy retained its gold standard status in the study [28]. In contrast, we found superior concordance between cytology and biopsy.
Limitation(s)
Follow up of all the patients could not be done in this study. The results of this study have to be compared with prospective studies of BAL for validation. As the year 2020 made people suffer with the pandemic due to Covid-19, BAL and the number of lung biopsies sent to the pathology laboratory were drastically reduced.
Conclusion(s)
Bronchoalveolar lavage as a diagnostic tool can be used for the diagnosis of various pulmonary conditions and also to obtain material for confirmation of the diagnosis. It is a less invasive technique which is preferred over needle biopsies and thoracoscopy. The data from the current study suggest that BAL differential cell counts per se contain diagnostic information of fundamental importance in frequently occurring non-neoplastic diseases in the community. Multi-centre studies are done which compare BAL with HRCT and histopathological pattern which may reduce the need for biopsies and provide information that can guide effective therapy. Future studies which investigate potential biomarkers in BAL that may predict the prognosis and response to therapeutic interventions shows that BAL has much more to contribute in the diagnosis of Non-neoplastic lung diseases.
TBLB: Transbronchial lung biopsy; BAL: Bronchoalveolar lavage
HPE: Histopathological evaluation; bold p-value indicates statistical significance