Introduction
Islet Amyloid Polypeptide (IAPP) is co-synthesised and co-secreted with insulin by islets of pancreatic β-cells, its increased concentration in individuals with Type 2 Diabetes Mellitus (T2DM), may involve in inflammatory processes.
Aim
To study and find the association of IAPP with inflammatory markers including high-sensitivity C-reactive protein (hsCRP), Tumour Necrosis Factor (TNF)-a and Interleukin (IL)-6 in patients with T2DM.
Materials and Methods
This was a cross-sectional study, conducted from, December 2015 to December 2019, on 262 subjects (30-60 years, 147 males and 115 females), including 131 healthy controls and 131 known cases of T2DM, Fasting blood glucose and glycated haemoglobin (HbA1c) was estimated by commercially available kit, and serum fasting insulin, IAPP, hsCRP, TNF-α, and IL-6 were analysed by ELISA. Insulin Resistance (IR) and insulin sensitivity were calculated. Statistical analysis was done with Statistical Package Social Sciences (SPSS) version 24. The descriptive statistics were expressed as Mean±SD, Student t-test was applied, and p≤0.05 was considered as statistically significant and highly significant at ≤0.01 at 95% CI. The relationship between IAPP and other variables were done by using Spearman Rank correlation (r).
Results
Circulatory level of IAPP was positively associated with serum hsCRP (r=0.312, p≤0.05) in T2DM. The elevated levels of IAPP (21.3±10.54 pmol/L), insulin (11.74±3.91 μU/mL), Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) (5.11±2.01) and inflammatory markers including hsCRP (3.44±1.77 mg/L), TNF-α (29.5±4.7 pg/mL) and IL-6 (20.2±5.2 pg/mL) p≤0.05 with poor glycaemic control (HbA1c 8.78±2.30%, p<0.001), and reduced insulin sensitivity {Quantitative Insulin Sensitivity Check Index (QUICKI) 0.31±0.02, p≤0.05} were compared with healthy control; indicates that hyperamylinemia may induce inflammation in individuals with T2DM.
Conclusion
Elevated degree of IAPP can be an important predictor and indicator of inflammatory processes and IR in uncontrolled diabetes.
Introduction
Diabetes Mellitus (DM) is recurrent metabolic disease [1], contribute to secondary pathological changes in vital organ systems and enormously raised burden on all diabetics and on health care system [2]. Global prevalence of diabetes was estimated 451 million by 2017, and it was expected to increase to 693 million by 2045 [3]. This elevated prevalence is observed in the developing countries like India [1]. It was estimated that diabetes will increase from 69.2 million (56.2-84.8 million) in 2015 to 123.5 million (99.1-150.3 million) by 2040 [2]. In 2017, diabetes-related expenses increased to US $ 850 billion worldwide, with nearly 5 million deaths attributed to diabetes [3].
Three primary factors are liable for advancement of T2DM: the first-diabetogenic factor is IR, considered as characteristic features of T2DM; where the observed effect of insulin is lower than anticipated. This condition is due to significant defect in the uptake of glucose induced by insulin, especially in glycogenesis, and a decreased degree of oxidation of glucose [4]. Insulin promotes energy utilisation and storage in addition to operation of other signalling pathways. Under the influence of an IR state, the body “starves in the midst of plenty”. With constant output of insulin, little or no utilisation, consequently, body get overwhelmed with insulin and glucose imbalance (hyperinsulinemia and hyperglycaemia, respectively) in presence of excess fatty acids. IR has emerged as key metabolic defect, leading to cluster of metabolic disorders like hyperglycaemia, hypertension, dyslipidemia, and obesity [5].
The second aspect leading to diabetes is environmental factors i.e., sedentary lifestyle, responsible for overweight/obesity. Central obesity associated IR is linked with a higher rate of cardiovascular disease than diabetic patients with normal weight (<18.5 to 24.9 kg/m2 BMI) [4]. The third diabetogenic factor is amyloidosis by IAPP also named as amylin, a 37 amino acid, release with insulin by pancreatic ß-cells in response to sugars, free unsaturated fats, and food intake [4]. Accumulating evidences, primarily from cellular and animal studies, proposed that IAPP plays significant role in controlling food intake, insulin action and energy homeostasis, as well as carbohydrate and lipid metabolisms at normal physiological limit. Further, numerous human studies have indicated cytotoxic consequences to vital organs, including the pancreas, heart, kidneys and brain [6]. The possible mechanism of amylin amyloid cytotoxicity include, elevated circulatory level of IAPP/amylin (hyperamylinemia), which may leads to accumulation [7], and causes conversion of soluble monomeric form into toxic non soluble oligomers and eventually fibrils [8]. These fibrils can form toxic nanoparticles, that has harmful effect on vital organs [9], therefore IAPP is considered as “amyloidogenic peptide”, and involved in development of T2DM [8].
The toxic amylin amyloid may thus play role in ß-cell dysfunction and ß cell death by activating pro-inflammatory reactions and inducing local islet inflammation [10]. An inflammatory toxicity may link to elevated redox stress, along with inflammatory biomarkers (including TNF-α, hsCRP, and IL-6), IR, metabolic syndrome, and early as well as late phase of T2DM [11]. TNF-alpha is an inflammatory cytokine that has been portrayed to assume an act in ß-cell demise and may likewise add to T2DM initiation. There are various examinations on the immediate impact of TNF-alpha, which forestalls insulin secretion [12]. Asian Indian phenotype including elevated IR and hsCRP are more vulnerable to progression of diabetes [13].
Liver-synthesised pentameric globular protein-hs-CRP, a non specific, acute stage reactant that acts as an effective long-term evaluation, so low-grade systemic inflammation can be used to predict the initiation of T2DM and Cardiovascular Diseases (CVDs) [14]. IL-6 is an inflammatory pleiotropic cytokine that is implicated in natural immune system processes, hematopoiesis, cardiovascular and other metabolic disease pathogenesis, facilitates inflammation under multiple pathological conditions [15]. The evidences are alarming, about the relationship between IAPP, inflammatory status and metabolic diseases like T2DM. Therefore, we focus on IAPP, a third diabetogenic factor. Present study was conducted to find the relationship between serum IAPP and inflammatory biomarkers including serum hs-CRP, TNF-α and IL-6 in Type 2 diabetic subjects.
Materials and Methods
The present cross-sectional study was carried out from December 2015 to December 2019 in Biochemistry Department, and Outpatient Department (OPD) of General Medicine and Diabetology, Mahatma Gandhi Mission Medical College and Hospital, Navi Mumbai, Maharashtra, India. Ethical approval was obtained from the Institutional Ethics Committee (IEC registration number- MGMIHS/RS/2015-16).
Sample size calculation: Based on previous study, the T2DM prevalence was 9.3% in Mumbai [16]. The sample size for present study was calculated as follows, using formula, N=Z2. P(1-P)/d2. Hence, minimum sample size required=130.54 (n=131) for T2DM and considered 1:1 ratio for healthy controls to study group (n=131).
Inclusion criteria: Total 262 subjects, aged 30 to 60 years, who attended the OPD during study time period were included in the study after taking the written informed consent. The 131 Diabetic patients among these subjects, diagnosed and confirmed as per World Health Organisation (WHO) and American Diabetes Association (ADA) criteria (HbA1c >6.5%) [17], formed the diabetic group while other 131 healthy subjects formed the control group.
Exclusion criteria: Patients with age <30 and >60 years, chronic renal disease, liver diseases, malignancy, metabolic bone disorders, and other inflammatory disorders were excluded from the study.
During history taking, the confounding factors like obesity, fever, dyslipidemia, fibroid disorders, atherosclerosis, arthritis, chronic illnesses and personal habits-like chronic smoking and alcoholics were taken into account for analysis of circulatory level of IAPP and to find its relationship with inflammatory markers in subjects with T2DM were ruled out and excluded.
Sample Collection
All participants were asked for 10 hours overnight fasting and on next day morning 08-10 mL of blood sample were collected under aseptic conditions by using Becton Dickinson (BD) vacutainer system. Of which 2.5 mL of blood sample was collected for fasting plasma glucose estimation in a fluoride bulb and it was analysed by glucose oxidase-peroxidase method on Beckman Coulter AU480 autoanalyser (Beckman Coulter, catalogue number: glucose-OSR6621), and for glycosylated haemoglobin (HbA1c) 2.5 mL blood was collected in Ethylene Diamine Tetra-acetic Acid vacutainer and analysed by High Performance Liquid Chromatography (HPLC) technique. A five mL of whole blood samples was collected in a plain bulb, after 30 minutes of collection; serum was separated out by centrifugation for five minutes at 3000 rpm. Serum samples were used to estimate the levels of IAPP, insulin, hsCRP, TNF-α, and IL-6 by using 96 well Enzyme Linked Immunosorbent Assay (ELISA kits) according to manufacturer’s instructions and read spectrophotometrically (RayBio, Chemux Bioscience, Cal-biotech, and Krishgen Bio-Systems resp.). IR was calculated by using homeostatic model (HOMA-IR), the formula is {HOMA-IR=Fasting insulin μIU/mL* fasting glucose (mmol/L)/22.5}, and insulin sensitivity (quantitative insulin check index) was calculated by using formula, QUICKI=1/(log (fasting insulin μU/mL) +log (fasting glucose mg/dL) [18].
Statistical Analysis
The analysis of data was carried out by using SPSS version 24.0. The relationship between IAPP and other variables were done by using Spearman Rank correlation (r). Descriptive Statistics expressed as Mean±Standard Deviation (SD), Student t-test was applied, and p≤0.05 was considered as statistical significant level and p≤0.01 for highly significant difference between the variables at 95% Confident Interval (CI).
Results
The average age of healthy subjects was 52.05±6.51 years and a type 2 diabetic subject was 53.3±6.75 years. [Table/Fig-1] shows age and gender wise distribution of healthy control and T2DM subjects, involved more number of males (n=147, 56%) than females (n=115, 44%) with maximum number of males from the age group of 51-60 years (n=68, 25.9%), followed by 41-50 years (n=42, 16%) and least in 30-40 years group (n=37, 14.1%). When compared, mean value of age among males and females from control and T2DM group, we could not found significant difference, whereas for gender results were mentioned as number of subjects in percent value.
Age and sex wise distribution of study population.
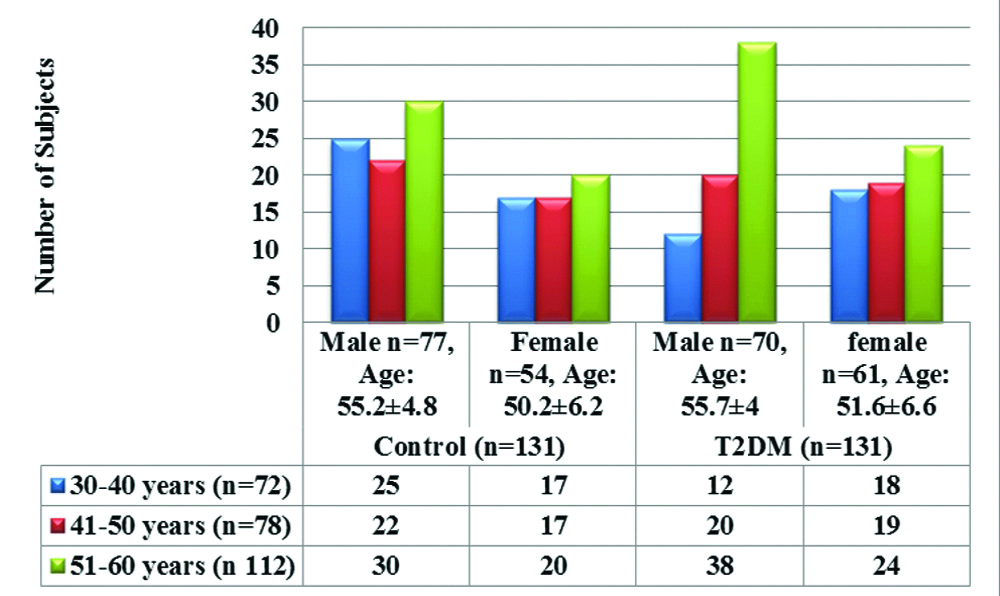
[Table/Fig-2] shows comparison of BMI and diabetic profile, the levels of Blood Sugar Fasting (BSL-F), and glycosylated haemoglobin (HbA1c) are significantly elevated (p≤0.001), and Body Mass Index (BMI), fasting insulin, IR (HOMA-IR) level significantly raised with p≤0.05, whereas insulin sensitivity (QUICKI) significantly decreased (p≤0.05) in T2DM as compared to healthy control.
Comparison of BMI and diabetic profile in healthy control and T2DM group.
| Variables | Control | T2DM | p-value |
|---|
| BMI (kg/m2) | 21.2±1.65 | 26.02±2.44 | ≤0.05* |
| BSL-F (mg/dL) | 82.9±8.21 | 175.73±35.3 | ≤0.01** |
| HbA1c (%) | 4.6±0.49 | 8.78±2.30 | ≤0.01** |
| Insulin (μU/mL) | 6.8±2.13 | 11.74±3.9 | ≤0.05* |
| HOMA-IR | 1.71±0.71 | 5.11±2.01 | ≤0.05* |
| QUICKI | 0.4±0.02 | 0.31±0.02 | ≤0.05* |
*p≤0.05 (significant) and **p≤0.01 (highly significant); T2DM: Type 2 diabetes mellitus; BMI: Body mass index; BSL-F: Blood sugar levels-fasting; HbA1c: Glycated haemoglobin; QUICKI: Quantitative insulin check index; HOMA-IR: Homeostatic model assessment of insulin resistance
[Table/Fig-3] shows highly significantly increased circulatory levels of serum IAPP and hsCRP (p≤0.01), whereas, significant increase in TNF-α and IL-6 with p≤0.05 in T2DM as compared to healthy control group.
Circulatory IAPP and inflammatory markers in study population.
| Variables | Control | T2DM | p-value |
|---|
| IAPP (pmol/L) | 10.68±3.72 | 21.3±10.5 | ≤0.01** |
| hsCRP (mg/L) | 0.67±0.34 | 3.44±1.77 | ≤0.01** |
| TNF-α (pg/mL) | 11.62±5 | 29.5±4.7 | ≤0.05* |
| IL-6 (pg/mL) | 8.0±3.13 | 19.2±5.2 | ≤0.05* |
IAPP: Islet amyloid polypeptide; hs-CRP: High-sensitivity C-reactive protein; TNF: Tumor necrosis factor; IL-6: Interleukin factor-6; *p-values are significant; **p-values highly significant
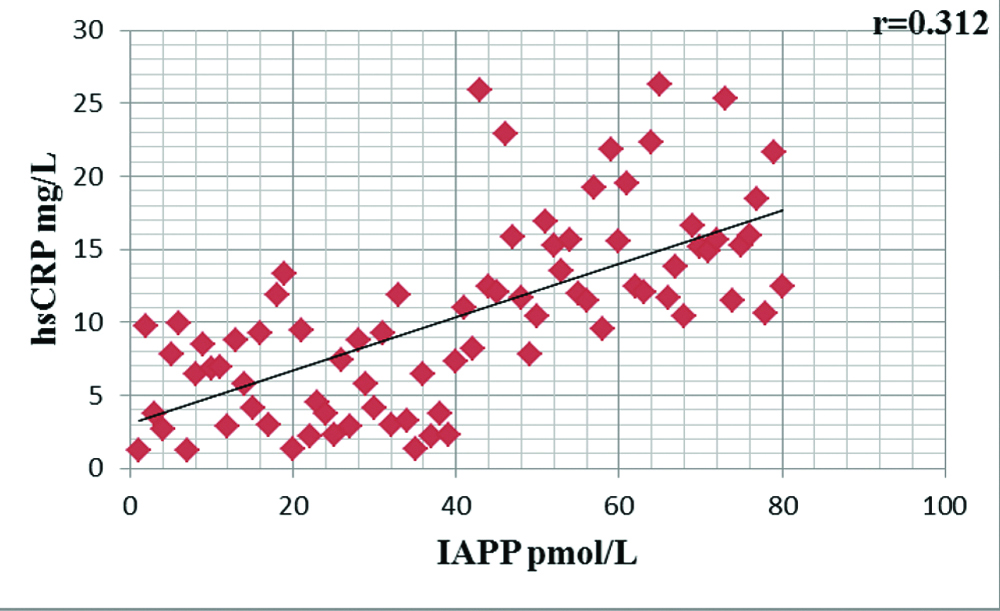
[Table/Fig-4] shows significant positive correlation between circulatory level of serum IAPP and inflammatory cytokine, serum hsCRP (r=0.312, p<0.05) in individuals with T2DM.
Correlation of IAPP with hsCRP in type 2 diabetes mellitus.
Discussion
Type 2 diabetes mellitus is related with marginal tissue IR. IR and abnormal hyperinsulinemia occur simultaneously with hyperamylinemia as well as with chronic low grade inflammatory mechanism. In present study, more number of males were involved than females particularly from the 51-60 years [Table/Fig-1]. These findings were in agreement with Srinivasan MP et al., they found that total n=50 males (66%) were predominantly high compared to female (n=26, 44%) [19].
In the present study, diabetes subjects are overweight with BMI is 26.02±2.44 i.e., >25 kg/m2 [Table/Fig-2], these finding are in accordance with Einarson TR et al., [20]. Overweight and obesity was highly prevalent in T2DM with higher cardiovascular risk. Studies in existing cardiovascular risk, i.e., asthma, diabetes, dyslipidemia, and rate of mortality is associated with total body weight/BMI [21].
The levels of diabetic profile are significantly increased in T2DM, as HbA1c (8.78±2.30%), and fasting blood glucose levels (175.73±35.3 mg/dL, p≤0.01); also fasting serum insulin (11.74±3.9 μU/mL) with IR (HOMA-IR: 5.11±2.01) p≤0.05. Whereas, significantly decreased insulin sensitivity (QUICKI: 0.31±0.02) p≤0.05 as compared to healthy control group (HbA1c: 4.6±0.49%, BSL-F: 82.9±8.21 mg/dL, insulin: 6.8±2.13 μU/mL, HOMA-IR: 1.71±0.71 and QUICKI: 0.4±0.02) [Table/Fig-2]. The American Diabetes Association and World Health Organisation (WHO) indicated that HbA1c is an index of previous three months glycaemic control for diagnosing diabetes mellitus. In systematic study and meta-analysis conducted by Cavero-Redondo I et al., presented summary of evidences, poor glycaemic control as a risk factor for mortality and cardiovascular risk [17].
QUICKI is a quantitative logarithmic translation of fasting plasma glucose level (mg/dl) and fasting serum insulin concentration (μU/mL), and it has better predictive potential and is an index of insulin sensitivity. The co-synthesis and co-secretion of Insulin with IAPP in reply to the nutritional stimuli; and hyperinsulinemia (IR) coincides with hyperamylinemia [11]. In present study [Table/Fig-3] significantly higher level of serum IAPP observed in diabetic population (21.3±10.5 pmol/L) as compared to healthy control group (10.68±3.72 pmol/L) with p≤0.01. O’Brien TD et al., showed an increased IAPP to insulin secretory ratio in islets derived from dexamethasone or glucose treated rats model as compared to islets isolated from fed and fasted rats, and also has been shown to elevate glucose concentration [22].
Studies showed that the amyloid plaques are a prominent risk factor for incidence as well as progression of type II diabetes and are present in 95% of DM cases [7,23]. Therefore, the amyloid forming process entails a number of phases, such as the initially increased amount of circulatory IAPP, which causes IAPP aggregation and deposition and transforms the toxic, insoluble fibrils, more prone to accumulate into toxic nanoparticle. This toxic form of islet amyloid causes apoptosis and destruction of β cells, this may lead to development of T2DM and also to islets inflammation due to amyloid induced beta cell destruction or apoptosis. In support to this hypothesis in present study, we observed significant positive association between circulatory concentration of IAPP with inflammatory cytokine, serum hsCRP (r=0.312, p<0.05) [Table/Fig-4] in diabetic population. Increased circulatory level of IAPP induces cytotoxicity even by small fibrils of trimers or tetramers; due to this it can easily and massively incorporated into cell membrane and contributes to formation of non selective pores [7].
Among inflammatory markers, we observed elevated levels of hsCRP (3.44±1.77 mg/L), TNF-α (29.5±4.7 pg/mL) and IL-6 (19.2±5.2 pg/mL) with increased level of IAPP (21.3±10.5 pmol/L) in diabetic patients [Table/Fig-3] as compared to healthy control (0.67±0.34 mg/L, 11.62±5 pg/mL, 8.0±3.13 pg/mL, and 10.68±3.72 pmol/L respectively), p≤0.01. These findings are at par with Hou X et al., who found strong positive association between IAPP and well-established risk factors of metabolic syndrome independently with obesity, IR, and inflammatory markers including hsCRP and IL-6 [6]. The elevated inflammatory markers in T2DM are likewise in agreement with Tangvarasittichai S et al., the most general marker for systemic inflammation is the hsCRP, demonstrates that inflammatory reactions responsible for pathogenesis of T2DM, and this provocative inflammatory status deteriorates in uncontrolled diabetes [24].
The pro-inflammatory cytokine- IL-6 triggers IR and T2DM through inflammatory processes via differentiation, migration, proliferation, and cell apoptosis. IL6 has detrimental impact by raising circulatory Free Fatty Acids (FFAs) and reducing the concentration of adiponectin [25]. The free unsaturated fats elevated by glucotoxicity and lipotoxicity in the islets and further raised TNF-a and IL-6 provoking impairment in islet cell function, lead to vicious cycle of inflammation [26]. Another proinflammatory, multi-potential cytokine TNF-a [25] level investigated, it initiates IAPP gene expression in murine pancreatic ß cells model and furthermore triggers IAPP through various stimulating pathways [27].
The present innovative analysis meant to measure the association of circulatory level of IAPP/amylin with inflammatory biomarkers in T2DM; these observations offer the novel target to drug inventors and new therapeutic approaches in DM. These findings may assist with comprehension and give additional routes of inflammation in diabetic condition. Future studies can be planned with more sample size and considering long term follow-up which will provide better outcome.
Limitation(s)
Association of amylin with inflammatory processes need to be understood in-depth in such chronic disease and for this purpose follow-up monitoring from the first day of disease diagnosed to the disease progression is require for at least five years, which is one of the limitations of the present study.
Conclusion(s)
The present study shows increased circulatory concentration of IAPP could be better predictor of inflammation and IR in patients with uncontrolled T2DM. This study reveals the significance of measurement of circulatory level of IAPP and inflammatory markers with routine diabetes panel to reduce the ensuing future risk in diabetic individuals. The study outcome gives new target to treat diabetic patients and provide an opportunity to develop novel treatment paradigms to reduce the incidence of future risk of diabetes related complications.
*p≤0.05 (significant) and **p≤0.01 (highly significant); T2DM: Type 2 diabetes mellitus; BMI: Body mass index; BSL-F: Blood sugar levels-fasting; HbA1c: Glycated haemoglobin; QUICKI: Quantitative insulin check index; HOMA-IR: Homeostatic model assessment of insulin resistance
IAPP: Islet amyloid polypeptide; hs-CRP: High-sensitivity C-reactive protein; TNF: Tumor necrosis factor; IL-6: Interleukin factor-6; *p-values are significant; **p-values highly significant
[1]. El-mesallamy HO, Suwailem SM, Seleem MM, Database AP, Visfatin and Apelin are new interrelated adipokines playing role in the pathogenesis of type 2 diabetes mellitus associated coronary artery disease in postmenopausal womenInternational Scholarly and Scientific Research & Innovation 2013 7(12):447-52. [Google Scholar]
[2]. Patil M, Kumar N, Nusrath A, Jayaram S, Rajeshwari A, Diabetes mellitus association of HbA1c with serum lipid profile and lipoprotein (a) in type 2 diabetes mellitusIJCRR 2014 06:20-25. [Google Scholar]
[3]. Atlas IDFD. Idf diabetes atlas. 2019 [Google Scholar]
[4]. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA, Association between insulin resistance and the development of cardiovascular diseaseCardiovasc Diabetol [Internet] 2018 17(1):01-14.Available from: https://doi.org/10.1186/s12933-018-0762-410.1186/s12933-018-0762-430170598 [Google Scholar] [CrossRef] [PubMed]
[5]. Ekpenyong CE, Relationship between insulin resistance and metabolic syndrome clusters: Current knowledgeRelatsh between Insul Resist Metab Syndr Clust Curr Knowl 2019 3(3):99-104. [Google Scholar]
[6]. Hou X, Sun L, Li Z, Mou H, Yu Z, Li H, Associations of amylin with inflammatory markers and metabolic syndrome in apparently healthy ChinesePLoS One 2011 6(9):01-08.10.1371/journal.pone.002481521935471 [Google Scholar] [CrossRef] [PubMed]
[7]. Despa S, Margulies KB, Chen L, Knowlton AA, Havel PJ, Taegtmeyer H, Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: A study in humans and ratsCirc Res 2012 110(4):598-608.10.1161/CIRCRESAHA.111.25828522275486 [Google Scholar] [CrossRef] [PubMed]
[8]. Pillay K, Govender P, Novel insights into amylin aggregationBiotechnol Biotechnol Equip 2014 28(1):123-35.10.1080/13102818.2014.90168026019498 [Google Scholar] [CrossRef] [PubMed]
[9]. Pillay K, Govender P, Amylin uncovered: A review on the polypeptide responsible for type II diabetesBiomed Res Int 2013 2013:82670610.1155/2013/82670623607096 [Google Scholar] [CrossRef] [PubMed]
[10]. Abedini A, Schmidt AM, Mechanisms of islet amyloidosis toxicity in type 2 diabetesFEBS Lett 2013 587(8):1119-27.10.1016/j.febslet.2013.01.01723337872 [Google Scholar] [CrossRef] [PubMed]
[11]. Hayden MR, Tyagi SC, Intimal redox stress: Accelerated atherosclerosis in metabolic syndrome and type 2 diabetes mellitusAtheroscleropathy. Cardiovasc Diabetol 2002 1:310.1186/1475-2840-1-312392600 [Google Scholar] [CrossRef] [PubMed]
[12]. Dunmore SJ, Brown JEP, The role of adipokines in β-cell failure of type 2 diabetesJ Endocrinol 2013 216(1):T37-45.10.1530/JOE-12-027822991412 [Google Scholar] [CrossRef] [PubMed]
[13]. Kumpatla S, Karuppiah K, Immaneni S, Muthukumaran P, Krishnan J, Narayanamoorthy SK, Comparison of plasma adiponectin & certain inflammatory markers in angiographically proven coronary artery disease patients with & without diabetes- A study from IndiaIndian J Med Res 2014 139(JUN):841-50. [Google Scholar]
[14]. Shaheer AK, Tharayil JK, Krishna PW, A comparative study of high sensitivity c-reactive protein and metabolic variables in type 2 diabetes mellitus with and without nephropathyJ Clin Diagnostic Res 2017 11(9):BC01-04.10.7860/JCDR/2017/30272.1052829207691 [Google Scholar] [CrossRef] [PubMed]
[15]. Qu D, Liu J, Lau CW, Huang Y, IL-6 in diabetes and cardiovascular complicationsBr J Pharmacol 2014 171(15):3595-603.10.1111/bph.1271324697653 [Google Scholar] [CrossRef] [PubMed]
[16]. Mohan V, Pradeepa RG, Epidemiology of diabetes in different regions of IndiaHeal Adm 2009 XXII(1&2):01-18. [Google Scholar]
[17]. Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V, Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysisBMJ Open 2017 7(7):01-11.10.1136/bmjopen-2017-01594928760792 [Google Scholar] [CrossRef] [PubMed]
[18]. Mahalle N, Garg MK, Naik SS, Kulkarni MV, Association of metabolic syndrome with severity of coronary artery diseaseIndian J Endocrinol Metab 2014 18(5):708-14.10.4103/2230-8210.13923825285291 [Google Scholar] [CrossRef] [PubMed]
[19]. Srinivasan MP, Kamath PK, Bhat NM, Pai ND, Manjrekar PA, Mahabala C, Factors associated with no apparent coronary artery disease in patients with type 2 diabetes mellitus for more than 10 years of duration: A case control studyCardiovasc Diabetol 2015 14(1):01-07.10.1186/s12933-015-0307-z26521236 [Google Scholar] [CrossRef] [PubMed]
[20]. Einarson TR, Acs A, Ludwig C, Panton UH, Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007-2017Cardiovasc Diabetol [Internet] 2018 17(1):01-19.Available from: https://doi.org/10.1186/s12933-018-0728-610.1186/s12933-018-0728-629884191 [Google Scholar] [CrossRef] [PubMed]
[21]. Gregory AB, Lester KK, Gregory DM, Twells LK, Midodzi WK, Pearce NJ, The relationship between body mass index and the severity of coronary artery disease in patients referred for coronary angiographyCardiol Res Pract 2017 2017:548167110.1155/2017/548167128512592 [Google Scholar] [CrossRef] [PubMed]
[22]. O’Brien TD, Westermark P, Johnson KH, Islet amyloid polypeptide and insulin secretion from isolated perfused pancreas of fed, fasted, glucose-treated, and dexamethasone-treated ratsDiabetes 1991 40(12):1701-06.10.2337/diab.40.12.17011756910 [Google Scholar] [CrossRef] [PubMed]
[23]. Chea J, Zhang S, Zhao H, Zhang Z, Lee EYC, Darzynkiewicz Z, Spatiotemporal recruitment of human DNA polymerase delta to sites of UV damageCell Cycle 2012 11(15):2885-95.10.4161/cc.2128022801543 [Google Scholar] [CrossRef] [PubMed]
[24]. Tangvarasittichai S, Pongthaisong S, Tangvarasittichai O, tumor necrosis factor-α, interleukin-6, c-reactive protein levels and insulin resistance associated with type 2 diabetes in abdominal obesity womenIndian J Clin Biochem 2016 31(1):68-74.10.1007/s12291-015-0514-026855490 [Google Scholar] [CrossRef] [PubMed]
[25]. Mahadik SR, Deo SS, Mehtalia SD, Role of adipocytokines in insulin resistance: Studies from Urban Western Indian PopulationInternational Journal of Diabetes and Metabolism 2010 18(1):35-42.10.1159/000497691 [Google Scholar] [CrossRef]
[26]. Agrawal NK, Targeting inflammation in diabetes: Newer therapeutic optionsWorld J Diabetes 2014 5(5):697-710.10.4239/wjd.v5.i5.69725317247 [Google Scholar] [CrossRef] [PubMed]
[27]. Cai K, Qi D, Wang O, Chen J, Liu X, Deng B, TNF-α acutely upregulates amylin expression in murine pancreatic beta cellsDiabetologia 2011 54(3):617-26.10.1007/s00125-010-1972-921116608 [Google Scholar] [CrossRef] [PubMed]