Changes in the morphology and chemical nature of the tooth surface is seen after bonding of the brackets [4]. Mutans Streptococci (MS) is supported by decalcifed enamel for its adhesion and proliferation. According to Zühlke A et al., and West JL et al., polymeric surface are prone to adhesion of living cells [5,6]. Polymers present in the composite resins may provide surface prone to adhesion and allow growth of the microorganisms, which are used for bonding of brackets. The fixed orthodontic appliances may enhance development and retention of bacterial plaque [7].
Information regarding the adhesion of bacterial species and plaque accumulation to bracket material is limited. Eliades T and Eliades G, concluded that in case of metallic brackets, there is an increased potential for microorganism attachment attributed to the fact that stainless steel presented the highest critical surface tension [8]. S. mutans adherence is higher in plastic or ceramic brackets than in metal brackets [9]. An in-vitro study revealed that Porphyromonasgingivalis and Escherichia coli lipopolysaccharide adherence was higher on stainless steel brackets when compared to ceramic, plastic and gold brackets [10].
The bacterial biofilm was significantly increased on the brackets, whereas, the Colony Forming Units (CFU’s) of Streptococcus gordonii, Porphyromonas gingivalis and Streptococcus mutans were not significantly increased on the tooth surface [12]. A recent in-vivo study also demonstrated that the microbial contamination in aligners was lower than that of metallic brackets, when used for a month and the labial fixed appliances showed less microbial contamination than the lingual fixed appliances followed by aligners [13].
Adequate information is needed in order to offer patients orthodontic treatment without significantly increasing their risk of developing white spots, caries, or gingival inflammation. The aim of this study was to evaluate and compare the adhesion properties of Streptococcus mutans to different brackets, in in-vitro, in the same circumstances as they are used clinically.
Materials and Methods
This in-vitro study was done in the Department of Orthodontics and Dentofacial Orthopaedics, K.D. Dental College and Hospital, Mathura, Uttar Pradesh in October 2016. Six groups of commercially available orthodontic brackets were used and each group contains 10 brackets. They were as follows:-
Group A- Metallic MBT Brackets (3M Unitek Gemini)
Group B- Metallic MBT Brackets (American Orthodontics)
Group C- Metallic MBT Brackets (Ormco)
Group D- Begg’s Brackets (TP Orthodontics)
Group E- Ceramic Polycrystalline MBT Brackets (Orthodontic Supplies Limited)
Group F- Self-ligating MBT Brackets (Orthodontic Supplies Limited)
Sample size calculation: The sample size was calculated according to the availability and feasibility of all the Six Bracket Systems. These 60 brackets were maxillary premolar brackets, 10 premolar brackets each of the six groups mentioned above. All brackets were ligated with elastomeric rings except for the 10 self-ligating brackets. The brackets were placed in sterile conditions in the cabinet of Laminar Flow according to their groups in their respective wells in the cell well culture plate [Table/Fig-1].
Brackets bonded in the cell well culture plate.

A diamond coated bur was used to roughen the floor of the plate and then brackets were placed at these exact places to bond the brackets, in such a way, that the bracket base will completely cover the roughened area. This was done so as to replicate the exact etching conditions which authors would have done while doing etching clinically on the surface of enamel as removal of smear layer occurs after it resulting in surface enamel roughness.
The brackets were then bonded with composite bonding material (3M UNITEK Transbond XT). The composite was applied to the bracket base so as to cover the entire mesh, and the bracket was pressed firmly onto the plate. The removal of excess of adhesive was achieved with the help of a number 23 Explorer also known as the “Shepherd’s Hook” Explorer by carefully removing excessive adhesive along the sides of the bracket base. Then the composite was light-cured for 30 seconds.
Preparation of Agar Plates
A 52 grams of brain heart infusion agar was mixed with 1 litre of distilled water. This mixture was heated in microwave oven and then autoclaved at 121°C for 15 minutes and left to cool to 50° C. A 25 mL of this agar was poured into petri dish and allowed to cool at room temperature [14].
Preparation of Bacterial Suspension
The Streptococcus mutans Strain MTCC 497 was procured from the Microbial Type Culture Collection and Gene Bank (MTCC) currently housed at the Council of Scientific and Industrial Research (CSIR) - Institute of Microbial Technology (IMTECH), Chandigarh. Streptococcus mutans strains from the lipolysed culture was taken from the microtube and transferred into test tube containing brain heart infusion broth and artificial saliva.
Hi media supplement Trypticase Yeast Cystiene Sucrose Bacto (TYCSB) was added to the mixture so as to inhibit growth of any microorganism other than Streptococcus mutans. This mixture was incubated overnight in a dessicator with 6% Carbon Dioxide (CO2) environment which was created by placing a candle in the dessicator [Table/Fig-2]. The optical density of the bacterial suspensions were adjusted to OD600=0.5.
Candle in the dessicator to produce 6% Co2.

This bacterial suspension was then exposed to the culture plates containing brackets and incubated for 24 hours at 37°C [14].
Sonication Done on Brackets
After incubation, plates were taken out from the incubator. Two times saline was used to wash the experimental setting in order to remove all non-adhered bacteria. Sterile pliers were used to randomly remove the brackets and flip-capped vial were used to put brackets which contained 1 mL of pre-Reduced Transport Medium (RTF). Sonication was used in order to detach the adherent bacteria by using four 30-second pulses with 30 intermittent cooling.
Preparation for Counting Colonies
Suspensions with phosphate buffer saline were then serially diluted upto 10-3 to 10-5 and 100 μL of each dilution was spread on brain heart infusion agar plate. These agar plates were placed inside dessicator with 6% CO2 environment and then incubated inside incubator for two days. Colonies were counted on colony counter. Colony counts were expressed as a CFU per unit area (cm2) of specimen.
Statistical Analysis
All the data collection was subjected to statistical analysis. The results were compared and analysed using Statistical Package for The Social Sciences (SPSS) software version 17.0 for Windows. For each bracket type, the mean and standard deviation of the CFU was calculated and the following statistical tests were applied: One-way analysis of variance (ANOVA) and Multiple comparisons Tukey’s test to determine the significance of differences among the means.
Results
[Table/Fig-3] demonstrated the results of one- way ANOVA between all six groups where the F-value came as 1383.024 with p-value <0.001 which is significant among all groups.
Comparison of Colony Forming Units (CFU) of different groups (One-way ANOVA).
| Groups | N | Mean | SD | F | p-value |
|---|
| A | 10 | 3.10 | 0.04 | 1383.024 | <0.001 |
| B | 10 | 3.82 | 0.11 |
| C | 10 | 3.46 | 0.13 |
| D | 10 | 1.52 | 0.24 |
| E | 10 | 5.60 | 0.21 |
| F | 10 | 7.53 | 0.24 |
p<0.05 is considered statistically significant, SD: Standard deviation
[Table/Fig-4] demonstrated the intergroup comparisons along with their p-values which reveal the level of significance. It was observed that: Group A was observed to be highly significant in comparison to groups D, E and F (p-value <0.001) and nonsignificant in comparison to groups B and C (p-value >0.05). Group B was highly significant in comparison to groups D, E and F (p-value <0.001) and nonsignificant in comparison to groups A and C (p-value >0.05). Group C was highly significant in comparison to groups D, E and F (p-value <0.001) and nonsignificant in comparison to groups A and B (p-value >0.05). Groups D, E and F were highly significant in comparison to other groups and to each other (p-value <0.001).
Multiple comparisons table demonstrating Intergroup comparisons with Tukey’s Analysis.
| (I) Group | (J) Group | Mean difference (I-J) | p-value |
|---|
| 3M (A) | American Ortho (B) | -0.719 | 0.067 |
| Ormco (C) | -0.358 | 0.059 |
| Begg’s (D) | 1.579 | <0.001 |
| Ceramic (E) | -2.507 | <0.001 |
| Self-Ligating (F) | -4.435 | <0.001 |
| American Ortho (B) | Ormco (C) | 0.361 | 0.06 |
| Begg’s (D) | 2.298 | <0.001 |
| Ceramic (E) | -1.788 | <0.001 |
| Self-Ligating (F) | -3.716 | <0.001 |
| Ormco (C) | Begg’s (D) | 1.937 | <0.001 |
| Ceramic (E) | -2.149 | <0.001 |
| Self-Ligating (F) | -4.077 | <0.001 |
| Begg’s (D) | Ceramic (E) | -4.086 | <0.001 |
| Self-Ligating (F) | -6.014 | <0.001 |
| Ceramic (E) | Self-Ligating (F) | -1.928 | <0.001 |
p<0.05 is considered statistically significant); p-value ≥0.5
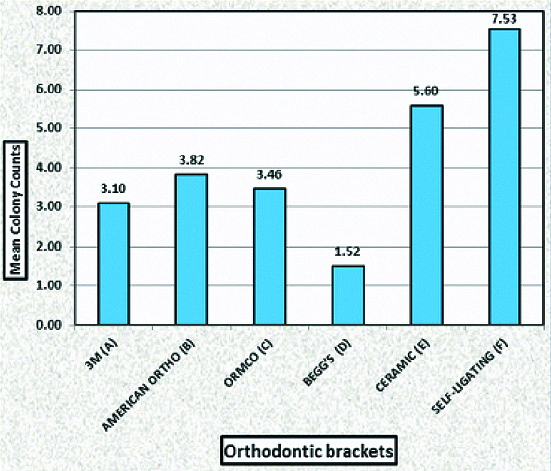
[Table/Fig-5] demonstrated the mean values of the CFU’s of all six groups. Group F expressed the highest mean value of colonies and Group D represented the least mean value of colonies.
This Graph demonstrates the mean values of the Colony Forming Units (CFU’s) of Streptococcus mutans in groups A-F.
Discussion
In the present study, it has been observed that the Begg brackets showed the least bacterial adhesion and the self-ligating brackets showed the highest bacterial adhesion and was statistically significant among all the groups (p<0.05). Ceramic brackets also showed a higher bacterial adhesion after the self-ligating brackets. Among the three groups of metallic brackets, 3M brackets showed the least bacterial adhesion but was statistically insignificant (p>0.05).
Enamel mineralisation or white spot lesions is a common side-effect of orthodontic treatment around orthodontic appliances. A favourable environment is created by the placement of orthodontic appliances for the accumulation of microorganisms, which causes exacerbation of any pre-existing caries. The incidence of enamel mineralisation after fixed orthodontic appliance can involve upto 50% of the patients [15].
Preventing these lesions has been an issue of worry for orthodontists because the lesions are not aesthetic, unhealthy, and potentially irreversible. Enamel mineralisation is caused by organic acids produced by MS. It was found that within dental plaque, there is an increase in the levels of MS with fixed orthodontic appliances, whereas, when the appliance is removed, levels of MS return to normal. During orthodontic treatment, mineralisation of enamel depends on MS adhesion to various orthodontic materials which play an important role. [15].
The orthodontic adhesive surrounding the brackets may act as a risk factor for enamel mineralisation as its rough surface provides an ideal site for the growth and attachment of oral microorganisms. In addition, orthodontic wires can play an important role in enamel mineralisation, because they provide additional adhesion sites for pathogenic bacteria, and their complex design impedes proper access to the tooth surface for cleaning. [11].
Various studies were done with models containing saliva coatings in order to simulate buccal conditions. [8,11,16,17]. Because of this, tested material properties and surface characteristics could be affected, which could inherent differences regarding the chemical composition of the material. However, these studies contain valid information and they reported that adhesion of Streptococcus mutans depends on the bracket material and have a characteristic pattern of adhesion. It was found that biofilm adhesion can be influenced by the saliva coating and the bacterial incubation time and these are the findings that are similar with those reported in the present study. Despite knowing these facts, recent studies gave rise to the development of a new approach by creating a modified in-situ model in order to reduce the artificiality of in-vitro studies by simulating a clinical situation, not found in other studies published [18].
In this study, among the six groups, that is, 3M metallic (Group A), American Ortho metallic (Group B), Ormco metallic (Group C), Begg (Group D), Ceramic (Group E) and Self-ligating (Group F), ceramic brackets (Group E) were in the high microbial adhesion group. Anhoury P et al., studied 32 metallic brackets and 24 ceramic brackets from orthodontic patients at the day of debonding and detected no significant differences between metallic and ceramic brackets with respect to caries-inducing Streptococcus mutans and Lactobacillus acidophilus counts [19]. Between metallic and ceramic brackets, there is a significant difference between additional species of mean counts of eight of 35 favouring one bracket type over the other with no definite pattern.
In this study, it indicates that adherence of Streptococcus mutans is weaker on metal than ceramic brackets. Fournier A et al., studied the affinity of Streptococcus mutans on metallic, plastic and ceramic brackets [9]. They concluded that the metal brackets present a lower potential for bacterial accumulation and have a lower cariogenic effect on the teeth than plastic and ceramic brackets. Gastel JV et al., also concluded that ceramic brackets have a significantly higher adhesion of Streptococcus mutans as compared to metallic brackets [17].
It is seen that bacterial adhesion is promoted by elastomeric rings when compared with metal ligatures. [17]. To compare clinical situations, the non self-ligating brackets were ligated with an elastomeric ring. In this study, it was found that adhesion of Streptococcus mutans between the different bracket systems indicate major differences.
In this study, self-ligating brackets did not show lower bacterial counts as compared with brackets ligated with elastomeric rings. For the metallic brackets (Groups A, B and C), it can even be concluded that the self-ligating brackets showed significantly higher (p<0.05) bacterial counts than were seen with classic brackets with elastomeric rings. The possible reason for this can be because the self-ligating brackets can provide an anaerobic condition in its bracket design mostly because of its spring clip resulting in development of anaerobic bacteria in addition to the aerobic bacteria as well. Gastel JV et al., studied Scanning Electron Microscopic (SEM) images on self-ligating brackets with several enlargement factors and it was found that different parts of the self-ligating brackets showed irregularities on the interfaces [17]. These parts seem to be welded together, as a result, self-ligating brackets may lead to formation of biofilm resulting in irregular surface.
Gastel JV et al., compared plaque formation on teeth bonded with self-ligating brackets and metallic brackets by means of an RCT with a split-mouth design with non bonded control teeth [20]. In that study, both anaerobe and aerobe CFU were significantly higher in teeth bonded with self-ligating brackets than in teeth bonded with metallic brackets. The shift from aerobic to anaerobic species was observed earlier in teeth bonded with self-ligating brackets than in teeth bonded with metallic brackets. The aerobe/anaerobe CFU ratio was also significantly lower in teeth bonded with self-ligating brackets than in teeth bonded with metallic brackets. In the clinical periodontal parameters, these differences were also visible.
In this study, Begg’s brackets (Group D) has the least CFU of Streptococcus mutans among all the groups indicating that Streptococcus mutans has the least adhesion property on these brackets. Metallic brackets had a higher rate on plaque adhesion than Begg brackets, hence, suggesting that bracket design could pose a potential effect on the bacterial load and periodontal parameters.
Moolya NN et al., compared over a period of seven days, teeth bonded with pre-adjusted and begg brackets with non bonded control sites via a de novo plaque growth for the undisturbed plaque formation [21]. This study suggested a rise in plaque and gingival index in both the pre-adjusted and begg site over a week. On the apical border of the brackets, however, the plaque accumulation was more on the pre-adjusted site. The reason could be larger surface area and complicated design of the bracket than the Begg type of brackets. Other reasons might be there could be higher binding force between high-surface energy and the bacteria and the selectivity in the bacterial adhesion. Pre-adjusted site harboured more anaerobic microflora as CFU ratio (aerobic/anaerobic) decreased significantly from day 3 to day 7 at preadjusted site [21] [Table/Fig-6].
Comparison of the present study with previous studies.
| Previous studies | Present study |
|---|
| Eliades T and Eliades G, concluded that in case of metallic brackets, there is an increased potential for microorganism attachment [8]. | In this study, the metallic brackets showed the least bacterial adhesion after begg brackets. |
| Fournier A et al., revealed in their findings that Streptococcus mutans adherence is higher in plastic or ceramic brackets than in metal brackets [9]. | In this study, it indicates that adherence of Streptococcus mutans is weaker on metal than ceramic brackets. |
| Anhoury P et al., detected no significant differences between metallic and ceramic brackets with respect to caries-inducing Streptococcus mutans and Lactobacillus acidophilus counts [19]. | In this study, it indicates that adherence of Streptococcus mutans is weaker on metal than ceramic brackets. |
| Gastel JV et al., found that different parts of the self-ligating brackets showed irregularities on the interfaces indicative of higher bacterial accumulation [17]. | In this study, the self-ligating brackets showed the highest bacterial adhesion. |
| Moolya NN et al., indicated an increase in the plaque accumulation on the apical border of Preadjusted brackets than begg brackets [21]. | In this study, it has been observed that the begg brackets showed the least bacterial adhesion. |
Limitation(s)
The sample size taken in this study was small due to non-feasibility of the bracket systems, and Only Streptococcus mutans was considered as microbiological entity in this study but not the other aerobic and anaerobic bacteria present in the oral cavity.
Conclusion(s)
This study proves that various orthodontic brackets serve as different loci for biofilm formation. It has also shown that Begg brackets are the most hygienic and self-ligating brackets are the most unhygienic among all the brackets in this study. Patients with self-ligating appliance are advised to maintain proper oral hygiene, regular brushing of teeth, use of floss and regular use of mouthwashes. Further research has to be done to improve the bracket design so that there should be less microbial adhesion on such type of brackets which are regularly used now-a-days.
p<0.05 is considered statistically significant, SD: Standard deviation
p<0.05 is considered statistically significant); p-value ≥0.5