Series of the proinflammatory process which incorporate the synthesis and release of cytokine, activations of complement neutrophils, and initiation of coagulation [7-9]. Many of the intermediates in the systemic inflammatory processes are serine proteases for example trypsin, chymotrypsin, thrombin, plasmin, coagulation factors kallikrein, neutrophil elastase, cathepsin and neutrophil protease-3 [10,11]. Through intercellular and intracellular signaling pathways, these proteases have an important role in regulation of inflammation. To nullify the effect of these proteases, several protease inhibitors are produced by the liver in the presence of sepsis, which include acute phase reactants such as alpha1-antitrypsin and proteins of the inter-alpha-inhibitor family [12].
The ULI, a urinary trypsin inhibitor is a significant protease inhibitor present in blood and urine of human [13]. With the help of neutrophil elastase, it cleaves the larger inter-alfa-trypsin inhibitor molecule in the presence of inflammation and plays a vital anti-inflammatory role [14-16]. There are various studies showing decrease in serum level of ULI in sepsis and septic shock [17,18]. Previously few studies suggested that the ULI may have role as a novel therapy in septic shock [17-19].
Also, low level of both thiamine and vitamin C are common in sepsis, combination of vitamin C, thiamine and corticosteroid also decreases the release of inflammatory substance leading to organ failure [20]. They also improve blood flow to vital organs and increases vasopressor response in septic shock [21].
Therefore, this study was conducted to compare the outcome of intravenous ULI (protease inhibitor known as urinary trypsin inhibitor) with the combination of Hydrocortisone, Ascorbic acid and Thiamine (HAT) therapy along with standard care in sepsis and septic shock.
Materials and Methods
This randomised controlled trial was conducted in the ICU of King George’s Medical University, Lucknow, India between July 2018 to June 2019 after Institutional Ethical Approval (registration number: ECR/262/Inst/UP/2013/RR-16).
Inclusion criteria: Adults, aged 18-60 years, with sepsis/septic shock, admitted to the ICU between July 2018 to June 2019 were eligible for enrollment into the study. Total 72 patients were included in this study, out of this 60 patients completed the study (30 received HAT and 30 received ULI) [Table/Fig-1].
CONSORT diagram of the study.

Exclusion criteria: Patients with less than 18 years, pregnant woman, active gastrointestinal haemorrhage, drug overdose, burn or trauma, requirement for immediate surgery, absolute neutrophil count <500 mm3, cluster of differentiation-4 (CD-4) <50/mm3, glucose-6-phosphate dehydrogenase deficiency, terminally ill patients in palliative care, known allergy or contraindication to one or more of the trial medications (Vitamin C, Thiamine, Hydrocortisone or ULI) were excluded from the study.
Sample size calculation: Calculated on the basis of variation in duration of hospital stay in the two groups calculated by using the formula:
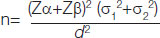
Where s1=7.1, The duration of hospital stay in first group; s2=7.2, The duration of hospital stay in second group [22]; d=min, the difference considered to be clinically significant; Type I error a=5% corresponding to 95% confidence level.
Type II error ß=10% for detecting results with 90% power of study
Data loss=10%.
The required sample size n=30 each group.
Computer operator divided 60 patients of sepsis in one of the two groups (30 in each) using computer generated random number table.
Group A (HAT): In this group, hydrocortisone was given according to the consensus guideline (50 mg six hourly) of the American college of critical care medicine, vitamin C was administered intravenously in a dose of 6 g/day in four equal doses, and thiamine was administered according to current recommendation in a dose of 200 mg 12 hourly. This triple regime was continued for five days [22].
Group B (ULI): Intravenous infusion of 2,00,000 International Units (IU) of ULI (U-Tryp) dissolved in 250 mL of 0.9% saline was given intravenous over one hour every 12 hourly for five days [23].
Blinding was done for allocation of the treatment (study medication) using serially numbered packages. Package numbered 1 was given to the first patient, and so on. Patients were randomised in a 1:1 ratio to receive metabolic resuscitation or ULI. Written informed consent was taken from the participants for inclusion in the study.
Study Procedure
Baseline demographic, clinical and laboratory data were recorded along with APACHE II and SOFA scores at the time of admission in ICU. Patient’s age, sex, and location along with clinical variables like temperature, resting heart rate, respiratory rate, mean arterial blood pressure as well as comorbid conditions were recorded at the time of admission and vitals were followed hourly till discharge or mortality.
All patients were subjected to baseline chest X-ray, complete blood count, serum procalcitonin, hepatic and renal function test, serum electrolyte, arterial blood gas and Prothrombin Time/international normalised ratio (PT/INR and followed daily. Two sets of blood culture (either both peripheral/one central and one peripheral) and culture of specimen from primary site of infection were sent before giving antimicrobials. (These vitals and parameters were used to calculate changes in APACHE II and SOFA scoring. These were not followed on individual basis).
In addition to the study medications, all patients were managed according to recent surviving sepsis guidelines 2016 [24]. Patients received antibiotics, intravenous fluids, vasopressors (noradrenaline, adrenaline, dopamine, or vasopressin), enteral or parenteral nutrition, transfusion of blood and blood products, and supportive care for organ dysfunction including mechanical or non invasive ventilation) as per the standard treatment protocols followed in each ICU.
Primary outcome of this study was to measure changes in SOFA score [25] from the day of admission to third and fifth follow-up day. Reduction in serum procalcitonin level, and lacate clearance rate were taken as secondary end point. Other variables like total length of ICU stay (from admission to discharge from ICU) and final outcome were also observed. The SOFA scoring, serum procalcitonin and serum lactate level were measured on first day before experimental drug administration followed by repeatition of same on third and fifth day. Patients were followed-up till 28 days after the start of treatment.
Statistical Analysis
All statistical analysis was done by using SPSS 21.0 windows software. All data were characterised in mean±SD and numbers (%). Comparisons between groups at different time intervals were assessed by using student t-test. The Chi-square test, Student’s t-test and analysis of variance (ANOVA) were used to analyse the data. A p-value less than 0.05 was considered significant.
Results
Demographic variables were comparable in both the group [Table/Fig-2]. There was no significant difference in mean APACHE II score between both the groups [Table/Fig-2].
Baseline characteristics in patients with sepsis.
| Baseline characterstics assessed | Group A (n=30) | Group B (n=30) | t-value/Chi-square | p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Age (years) | 36.7±12.5 | 37.5±12.9 | | 0.70 |
| APACHE II | 16.87±3.34 | 18.52±3.14 | -1.96 (t-test) | 0.055 |
| Sex | n (%) | n (%) | | |
| Female | 13 (43.3%) | 9 (30.0%) | 1.15(Chi-square test) | 0.284 |
| Male | 17 (56.7%) | 21 (70.0%) | | |
Group A: HAT: Hydrocortisone, Ascorbic acid and Thiamine
Group B: ULI: Ulinastatin; p-value calculated from Unpaired ‘t’ Test
The mean procalcitonin level was comparable in both the groups at the time of admission [Table/Fig-3]. While, it was significantly lower in group B as compared to group A on day 3 and day 5. On intragroup comparison, in group A, reduction in mean serum procalcitonin level was not significant from day 1 to day 5, whereas, in group B, there was significant reduction of mean procalcitonin level from day 1 to day 5 [Table/Fig-3].
Comparison of procalcitonin levels and SOFA Score in Intergroup and intragroup.
| Clinical assessments | Group A (n=30) | Group B (n=30) | t-value | 1p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Procalcitonin level |
| Day 1 | 78.77±86.78 | 46.34±82.87 | 1.47 | 0.148 |
| Day 3 | 76.02±78.52 | 25.56±51.32 | 2.91 |
| Day 5 | 104.16±113.03 | 20.10±61.15 | 3.54 |
| Intragroup | F=2.364, 2p=0.103 | F=5.292 | |
| SOFA score |
| Day 1 | 12.53±3.14 | 13.83±3.28 | -1.55 | 0.127 |
| Day 3 | 12.03±4.77 | 7.76±3.67 | 3.85 |
| Day 5 | 12.10±7.36 | 4.79±4.02 | 4.71 |
| Intragroup | F=0.200, 2p=0.819 | F=72.878 | |
| Lactate |
| Day 1 | 4.65±3.07 | 1.93±0.77 | 4.64 | <0.001* |
| Day 3 | 4.40±3.47 | 1.42±0.44 | 4.59 |
| Day 5 | 5.00±4.56 | 0.89±0.37 | 4.84 |
| Intragroup | F=1.138, 2p=0.328 | F=31.792 | |
1p-value calculated from Unpaired ‘t’ Test; 2p-value calculated by ANOVA
SOFA: Sequential organ failure assessment;
Group A: HAT therapy
Group B: ULI therapy
The mean SOFA Score was higher in group B (13.83±3.28) as compared to group A (12.53±3.14) but the difference was not found to be significant (p=0.127) at the time of ICU admission [Table/Fig-3]. The change in mean SOFA Score was not significant in group A from day 1 to day 5 while there was significantly reduction in group B from day 1 to day 5 [Table/Fig-3]. Clearance rate of serum lactate was significant in group B as compared to group A from day 1 to day 3 and day 5 as shown in [Table/Fig-3] while, reduction in mean lactate level was not significant in group A from day 1 to day 5.
The duration of ICU stay (days) was comparable in both the groups as shown in [Table/Fig-4]. There was significant mortality benefit in group B (20%) as compared to group A (50%) [Table/Fig-4].
Comparison of duration of ICU stays between the groups.
| Variables assessed | Group A (HAT) (n=30) | Group B (n=30) | t-value/Chi-square | p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Duration of ICU stay (days) | 12.43±6.88 | 12.07±5.18 | 0.23 | 0.819 |
| Outcome | n (%) | n (%) | | |
| Discharged | 15 (50.0%) | 24 (80.0%) | 5.93 | 0.015 |
| Expired | 15 (50.0%) | 6 (20.0%) |
Discussion
The fundamental treatment of patients with sepsis was similar in both the groups except both group regimes. The treatment/management and transfer forms provided to patients during ICU stay were according to institutional protocols. Haemodynamics were maintained by giving vasopressors and crystalloids. Norepinephrine was the vasopressor of first choice and was titrated to achieve a MAP >65 mmHg.
In this study, the mortality was significantly higher (50%) in HAT group (group A) as compared to ULI group (group B). This study is supported by previous studies [23,26] showing use of ULI was associated with reduced mortality rate in 28 days after ICU admission. Karnad DR et al., (2014) showed that 28 day mortality was 7.3% with ULI (4 deaths) versus 20.3% (12 deaths) with placebo (p=0.045) [23]. While, Balakrishnan M et al., (2018) found that early use of hydrocortisone together with ascorbic acid and thiamine, intravenously, prove to be effective in the reduction of vasopressors dosage and mortality of patients with severe sepsis and septic shock [26].
Procalcitonin is very useful for early diagnosis of sepsis as well as to monitor the antimicrobial treatment regimen. That is why, this biomarker as a significant prognostic indicator of sepsis. On intergroup comparison, on day 3 and 5, there was a significant reduction of procalcitonin in group B (ULI). On intragroup comparison, rate of reduction of procalcitonin level was also found to be significant in group B (p=0.008) as compared to group A (p=0.103). Wang Y (2018) also found that addition of ULI can significantly improve 60-day prognosis and reduce serum PCT level in severe sepsis patients [27]. Xu Q et al., (2019) found that PCT level dropped to a greater extent (changes of PCT on day 3 (ng/mL), median-5.5 (-7.4 to -3.2 p-value 0.03) in the ULI group [28]. While Balakrishnan M et al., (2018) reported that the Procalcitonin (PCT) levels were significantly lower in treatment group (HAT) as compared to control at day 3 (68.11±33.64 vs 33.2±27.55 ng/mL; p-value=0.0161) and day 4 (70.03±29.74 vs 26.3±23.08 ng/mL; p-value=0.0009) [26].
The mean SOFA score improvement in organ dysfunction is rapid in group B (ULI). Another study compared that SOFA score between survivors and non survivors (SOFA: 11 for non survivors, 7 for survivors; p-value <0.001) after using ULI in critically ill patient which showed significant reduction of SOFA score in ULI group [27] in another study by Balakrishnan M et al., (2018) showed no difference in the SOFA score and mortality benefit between the studied groups [26].
Change in lactate level showed significant reduction in group B (ULI) as compared to group A (HAT) (p=0.328). Previously a study showed that the arterial blood lactate was significantly lower in the observation group as compared to control group after using ULI in Acute Respiratory Distress Syndrome (ARDS) patient [29]. Litwak JJ et al., (2019) using vitamin C, hydrocortisone, and thiamine combination therapy as a standard of care in patients with septic shock found that there was significant reduction in serum lactate level [30].
In this study, difference in the length of ICU stay was not significant between both the groups (12.45±6.88 vs 12.07±5.18). Vincent JL et al., showed mean hospital stay was less in the ULI group by an average of 12.4 days (p=0.001) as compared to placebo [25]. In this study, ULI and combination of vitamin C, hydrocortisone and thiamine is effective in septic shock patient when used in early stage of septic shock but ULI found to be better in comparision.
Limitation(s)
The present study is single-centered small study. Presentation of patients in septic shock to our centre was delayed so exact timing of intervention with ULI could not be assessed properly.
Conclusion(s)
This study demonstrated that the early use of injection ULI leads to better outcome in septic shock. There is significant reduction in new onset, organ dysfunction may indicate a potential role of ULI in changing the progression of this serious conditions and could alter the pathophysiology of disease. A larger multicentric randomised controlled study is needed to further confirm the survival benefit seen in the present study and also to investigate its mechanism of action in humans.
Group A: HAT: Hydrocortisone, Ascorbic acid and Thiamine
Group B: ULI: Ulinastatin; p-value calculated from Unpaired ‘t’ Test
1p-value calculated from Unpaired ‘t’ Test; 2p-value calculated by ANOVA
SOFA: Sequential organ failure assessment;
Group A: HAT therapy
Group B: ULI therapy