Glaucoma is the second most common cause of blindness worldwide. In India, approximately 11.2 million adults above 40 years are estimated to have glaucoma; 6.48 million with POAG and 2.54 million with PACG [1]. Glaucoma is an irreversible optic neuropathy with corresponding visual field defects which progress to blindness unless timely intervention is done. An important risk factor is the level of IOP. It is determined by the aqueous humour drainage which in turn depends on the status of angle of anterior chamber. Angle structures are occluded in PACG whereas they are open in POAG although there is sclerosis of trabecular meshwork.
Goldmann Applanation Tonometry (GAT) has been considered as the most accurate method for IOP measurement due to its low intra- and inter-observer variability [2]. Gonioscopy allows us to examine the angle of anterior chamber and forms part of complete ophthalmic examination and it is mandatory for the diagnosis and management of glaucoma. Gonioscopy permits the identification of eyes at risk for closure and detects angle abnormalities [3].
Since visual field loss is the morbid sequalae, it should be evaluated and graded to understand the severity of disease. Humphrey Field Analyser (HFA) is used for this purpose which employs the principle of static perimetry. The Full Threshold strategy is the standard technique in static threshold perimetry and used in most glaucoma-related clinical trials, which is followed here [4].
A study in South-east Asia suggested a stronger correlation between initial IOP which was recorded before starting treatment and the severity of visual field loss in PACG than POAG. This indicates that IOP may be more important as a causative factor for optic nerve damage in PACG than it is in POAG [5]. Since, IOP is the most important modifiable risk factor to halt the progression of optic neuropathy and consequent visual field loss, it was studied in the present population. The disease pathology in PACG is likely to be entirely dependent on intraocular pressure whereas it is unlikely in POAG [6]. While IOP-lowering is a mainstay of glaucoma therapy, and a very successful “neuro-protectant” in itself, complementary approaches to glaucoma therapy is essential to prevent visual deterioration [7]. The study was done to assess the correlation between IOP and visual field loss in PACG and POAG.
Materials and Methods
This cross-sectional observational study of newly diagnosed cases of PACG and POAG above 40 years was done in Regional Institute of Ophthalmology, Trivandrum, Kerala, India over a period of one year from April 2016 to May 2017. After obtaining clearance from Institutional Ethics Committee (IEC no/49/16/HEC/RIOTVPM) the study was done according to following criteria.
Inclusion criteria: Newly diagnosed cases of PACG and POAG above 40 years who attended glaucoma clinic for complete ocular examination and visual field analysis were included in the study after obtaining the informed consent.
Exclusion criteria: Secondary glaucomas, acute congestive glaucoma, patients on anti-glaucoma medications and topical steroids and those with history of ocular surgery or trauma in the affected eye. Patients with refractive error higher than 5D myopia or hypermetropia and unreliable visual fields even after repeated testing were also excluded.
Sample size calculation: Sample size is calculated using the formula
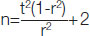
where r is the Pearson correlation coefficient. t=(z1-α/2+z1-β)=√7.84
α=5%, β=20%
In PACG, sample size was 21, according to r value of 0.41 for AGIS score and significance level of 95%. In POAG, sample size calculated as 85, according to r value of 0.21 for MD and significance level 95% based on a reference study [5].
Procedure
Patients whose IOP was recorded more than 21 mmHg in either eye was evaluated further by following examinations and those who satisfy inclusion criteria selected for study. Their age, gender, ocular history, medical history and family history of glaucoma were noted. The Best Corrected Visual Acuity (BCVA) assessed using Snellens chart and baseline IOP was recorded with GAT. Colour vision was recorded using Ishihara chart. The calibration of tonometer was done by glaucoma surgeon on weekly basis. A slit lamp, biomicroscopy and indentation gonioscopy using posner lens was done. Gonioscopy was done at a dim illumination with a very narrow slit. Care was taken to avoid light in the pupillary area. The angle structures were examined at 16 times magnification. An occludable angle was defined as one in which the posterior pigmented trabecular meshwork was seen less than 90 degree of angle without indentation [8].
Optic nerve head and Retinal Nerve Fibre Layer (RNFL) was assessed using 90 D lens and cup disc ratio was estimated. Enlargement of vertical cup disc ratio above 0.6 or asymmetry of 0.2, presence of bayonetting, laminar dot sign, beta peripapillary atrophy, nasal shift of vessels and baring of circumlinear vessels were suggestive of glaucomatous optic neuropathy, hence their visual fields were evaluated [9].
Two reliable visual field test were done using HFA Swedish Interactive Threshold Algorithm (SITA) standard, 24-2 pattern (with full threshold strategies, fixation loss less than 20%, false positive and false negatives less than 33%). Refractive correction is used during HFA. The more reliable of two baseline visual field is selected. A size III white stimulus with the foveal threshold test turned on was used. The pupil diameter was more than 2 mm. If necessary, the pupil was dilated before the test. An appropriate age-related plus-power lens was added to the distance refraction for best-corrected vision. The room lights were dimmed without direct light falling on the patient. The patients were allowed to blink and to maintain fixation. Test results were checked for reliability. If the test was unreliable, it was repeated.
The Mean Deviation (MD) reflects overall depression of field, with normal values between 0 to -2 dB. The values of the test are added and divided on the number of test locations. Thus the mean value of the test is obtained.The difference between this value and normal represents MD [10]. Pattern standard deviation is a measurement that indicates a difference in the sensitivity of adjacent tested points. In glaucoma patients as irregular depression of visual field sensitivity progresses, PSD values increase [11]. Severity of visual field loss was scored using AGIS scoring system [12]. In the AGIS scoring system, the 24-2 area of the visual field was divided into three areas: the nasal, superior hemifield and inferior hemifield areas. The amount of sensitivity loss (total deviation) necessary to be considered abnormal varies from 5 to 9 dB depending on the location. A nasal defect is a group of three or more contiguous depressed points that may cross the horizontal midline. A nasal step is one contiguous point or more in the superior nasal area without any depression in the opposite nasal area and vice versa. Any one defect results in a score of 1. If four or more of the nasal test points have defect depths of 12 dB or more, a score of 2 is marked. The superior and inferior hemifields are scored separately. The number of groups of three or more contiguous depressed points is identified and the total number of points within these groups was summed. A score of one for a total of 3 to 5 points, two for 6 to 12 points, three for 13 to 20 points, and four for more than 20 points were given. Additional scores are given according to the defect depth. A score of 1, 2, 3, 4, or 5 is added respectively if atleast half the points are depressed by ≥12, ≥16, ≥20, ≥24, or ≥28 decibels. A maximum score of 9 is given to each hemifield, and a maximum score of 2 to the nasal area. Thus resulting in a total score that can range from 0 (no field loss) to 20 (end stage) [13].
The PACG was diagnosed when the angle was occludable and there was visual field loss according to AGIS scoring system; POAG was diagnosed when they had open angle on gonioscopy with glaucomatous visual field loss and no apparent secondary cause. Anterior Chamber Depth (ACD) and Axial Length (AL) measurement was done with ultrasound A scan; central corneal thickness was measured using pachymeter.
Statistical Analysis
Data analysis was done for the eye with higher IOP and the more reliable visual field was selected. It was carried out using SPSS version 16. Baseline demographic and clinical data were presented as mean±SD. Linear regression analysis was used to assess the correlation between baseline IOP with MD, PSD and AGIS score data. Since values didn’t follow normal distribution, Spearman Correlation was used.
Results
A total of 110 patients were enrolled in the study; 25 belonged to PACG group and 85 were POAG patients. In both the PACG and POAG group, maximal incidence was between 5th and 7th decade with mean age of 58.72±10.07 years in PACG and 60.28±10.42 years in POAG. The gender difference was less in PACG 52% and in POAG 52.9% were males [Table/Fig-1]. Co-existent diabetes mellitus and hypertension were more in POAG group (40% vs 47.05%; 32% vs 48.24%) [Table/Fig-2].
Demographic parameters of all subjects.
| Demographic variables | PACG group (n=25) | POAG group (n=85) |
|---|
| Age (years) | Frequency (n) | Frequency (n) |
| ≤55 | 9 | 28 |
| 56-70 | 13 | 42 |
| >70 | 3 | 15 |
| Sex ratio |
| Male:female | 13:12 | 45:40 |
| Total | 25 | 85 |
PACG: Primary angle closure glaucoma; POAG: Primary open angle glaucoma
Co-morbidities in subjects.
| Type | N | DM | HTN |
|---|
| PACG | 25 | 10 | 8 |
| POAG | 85 | 40 | 41 |
DM: Type 1 Diabetes mellitus; HTN: Systemic hypertension
A positive family history was considerably higher in POAG as compared to PACG group (41.18% vs 16%) [Table/Fig-3].
Data of positive family history.
| Family history of glaucoma | PACG (n) | POAG (n) |
|---|
| Yes | 4 | 35 |
| No | 21 | 50 |
| Total | 25 | 85 |
The distribution of BCVA and colour vision in PACG and POAG is shown in [Table/Fig-4]. The mean AL was shorter in PACG (21.59±1.15 vs 23.55±0.79 mm) with shallower mean ACD (2.30±0.44 vs 2.57±0.28 mm) [Table/Fig-5,6].
Distribution of cases according to visual impairment; Best Corrected Visual Acuity (BCVA) and colour vision.
| Visual analysis | PACG (n) | POAG (n) |
|---|
| BCVA |
| 6/6 | 4 | 19 |
| 6/9 | 4 | 23 |
| 6/12 | 7 | 20 |
| 6/18 | 2 | 14 |
| 6/24 | 3 | 4 |
| 6/36 | 3 | 4 |
| 6/60 | 2 | 1 |
| Colour vision |
| Normal | 20 | 72 |
| Defective | 5 | 13 |
(n=25 in PACG group; n=85 in POAG group)
Ocular biometrics assessment in PACG.
| PACG | N | Minimum | Maximum | Mean | SD |
|---|
| Age (years) | 25 | 40 | 79 | 58.72 | 10.07 |
| IOP (mm Hg) | 25 | 29 | 42 | 33.56 | 3.40 |
| CCT (mm) | 25 | 501 | 569 | 531.96 | 14.15 |
| ACD (mm) | 25 | 1.71 | 3.75 | 2.30 | 0.44 |
| AL (mm) | 25 | 20.08 | 23.92 | 21.59 | 1.15 |
CCT: Central corneal thickness; ACD: Anterior chamber depth; AL: Axial length
Ocular biometrics assessment in POAG.
| POAG | N | Minimum | Maximum | Mean | SD |
|---|
| Age (years) | 85 | 40 | 84 | 60.28 | 10.42 |
| IOP (mm Hg) | 85 | 25 | 43 | 31.50 | 3.50 |
| CCT (mm) | 85 | 0.476 | 605 | 504.60 | 129.29 |
| ACD (mm) | 85 | 2.03 | 3.25 | 2.57 | 0.28 |
| AL (mm) | 85 | 21.82 | 25.38 | 23.55 | 0.79 |
Pearson correlation for linear regression for baseline IOP and AGIS score in PACG demonstrated correlation coefficient (r) as 0.805, with p-value <0.001. Hence, the correlation is significant and positive [Table/Fig-7]. No such correlation was noted with regard to PSD. Since p-value is >0.05, the correlation is not significant for MD and PSD in POAG [Table/Fig-8].
Correlation of IOP with visual field scores in PACG.
| Correlation of IOP with other variables (PACG) | N | Mean | SD | Pearson correlation coefficient (r) | p-value* |
|---|
| MD (dB) | 25 | -14.36 | 7.57 | 0.812 | <0.001 |
| PSD (dB) | 25 | 8.46 | 3.66 | -0.158 | 0.450 |
| AGIS | 25 | 10.44 | 5.28 | 0.805 | <0.001 |
| CCT (mm) | 25 | 531.96 | 14.15 | -0.233 | 0.262 |
| ACD (mm) | 25 | 2.30 | 0.44 | 0.013 | 0.949 |
| AL (mm) | 25 | 21.59 | 1.15 | -0.019 | 0.929 |
Sig 2 tailed test*. IOP: Intraocular pressure; MD: Mean deviation; PSD: Pattern standard deviation; AGIS: Advanced glaucoma intervention score; IOP mean=33.56 mmHg, SD=3.40 mmHg
Correlation of IOP with visual field scores in POAG.
| Correlation of IOP with other variables (POAG) | N | Mean | SD | Pearson correlation coefficient (r) | p-value* |
|---|
| MD (dB) | 85 | -16.27 | 9.61 | -0.058 | 0.597 |
| PSD (dB) | 85 | 8.12 | 4.20 | 0.071 | 0.518 |
| AGIS | 85 | 11.85 | 5.65 | 0.026 | 0.816 |
| CCT (mm) | 85 | 504.60 | 129.29 | -0.055 | 0.616 |
| ACD (mm) | 85 | 2.57 | 0.28 | 0.122 | 0.266 |
| AL (mm) | 85 | 23.55 | 0.79 | 0.072 | 0.511 |
Sig 2 tailed test*, IOP: Intraocular pressure; MD: Mean deviation; PSD: Pattern standard deviation; AGIS: Advanced glaucoma intervention score; IOP mean=31.5 mmHg, SD=3.50 mmHg
Discussion
An awareness of modifiable and non-modifiable risk factors regarding glaucoma is essential since it is a major cause for blindness worldwide. In present study, most of the patients belong to the age group of 56-70 years. Advancing age is a risk factor for glaucomas [14-16]. This correlates well with the study conducted by Markowitz SN and Morin JD, who also found that there is increased prevalence of pupillary block glaucomas between 50-70 years of age [17]. Because anterior chamber volume/area is the volume/area bounded by the cornea, the iris, and the anterior surface of the lens, the change in anterior chamber area/volume is the composite result of changes in these tissues. As age advances, thickness of lens increases and thus may be an important factor causing the age-related diminution of these parameters [18].
There was no significant gender predisposition observed in the study probably due to the selection criteria of reliability of visual fields rather than incidence. In ocular hypertension study, male gender was found by univariate analysis to be a useful predictor for the onset of POAG [19,20].
A positive family history of glaucoma was noted in 16% of PACG patients and 41.18% of POAG patients. A study by Awadalla MS et al., demonstrated that around 50 percent of POAG patients have a positive family history and their first degree relatives had an approximately nine fold increased risk of developing glaucoma [19]. This study showed that a family history of glaucoma is associated with the presence and severity of PAC and POAG [21].
In the South Indian population screened, siblings of angle-closure patients had a greater than one in three risk of prevalent angle closure, whereas siblings of PAC/PACG patients had a >10% risk of prevalent PAC/PACG. Hence screening siblings of angle-closure patients is likely to detect more cases of angle closure [22]. The contribution of genetics in glaucoma risk prediction limited to the knowledge of family history warrant evaluation in siblings and for early diagnosis [14].
Systemic Hypertension has been noted as a potential risk factor for glaucoma in clinic based studies [23]. Patients using medications for hypertension is also exposed to hypotension and resultant decreased ocular perfusion pressure with IOP, which increases vulnerability to optic nerve damage in open angle glaucoma.
Linear regression analysis demonstrated a significant positive correlation observed between IOP and AGIS score (correlation coefficient, r=0.805 where p<0.001, in PACG group). This suggests that IOP is strongly implicated as a causative factor for optic nerve damage in PACG. This is comparable to the results obtained in the study, IOP and visual field loss in primary angle closure and POAG by Gazzard G et al., which demonstrated significant correlation between IOP and AGIS score in PACG [5].
There was strong positive correlation between IOP and MD where correlation coefficient r=0.812 and p<0.001. Correlation between MD and the more specific AGIS score implies that visual field loss was glaucomatous. Correlation between IOP and PSD was not found statistically significant. In the present study, we have demonstrated a strong correlation between IOP before initiation of treatment and the severity of visual field loss in PACG patients. These measures were based on parameters adjusted according to age related decrease in retinal sensitivity; hence age distribution do not affect the result. In both the groups, patients with miosis and significant cataract were not included in the study. This indicates difference in field severity is not contributed by lens opacity or miosis.
The risk for blindness was also much higher for angle closure glaucoma than for POAG and therefore the benefit is greater for each case of angle-closure glaucoma prevented [24-27]. In POAG, linear regression analysis demonstrated that the correlation between IOP and AGIS score was not statistically significant since p value is more than 0.05. Similarly, MD and PSD did not show significant correlation with IOP. This suggests that IOP alone cannot be considered as a causative factor for optic nerve damage in POAG and also supports the multifactorial etiology of optic nerve damage in POAG. This is comparable to the results obtained in the study, IOP and visual field loss in primary angle closure and POAG by Gazzard G et al., which demonstrated no significant correlation between IOP and AGIS score in POAG. In the present study, we found out that IOP alone was insufficient to explain the severity of visual field loss in POAG hence other modifying factors were more important in POAG [5].
Limitation(s)
Since the study subjects were selected on the basis of reliability of visual field test and other criteria, the study population was not representative of normal population. So the demographic characteristics could not be generalised to normal population. A bias may be created by difference in the number of subjects in POAG and PACG.
Conclusion(s)
The significant correlation between IOP and visual field implies that IOP is a modifiable risk factor in PACG. High incidence of positive family history in POAG highlights the importance of glaucoma screening and follow-up in first degree relatives of POAG patients. The multifactorial pathogenesis of POAG should be further explored.
PACG: Primary angle closure glaucoma; POAG: Primary open angle glaucoma
DM: Type 1 Diabetes mellitus; HTN: Systemic hypertension
(n=25 in PACG group; n=85 in POAG group)
CCT: Central corneal thickness; ACD: Anterior chamber depth; AL: Axial length
Sig 2 tailed test*. IOP: Intraocular pressure; MD: Mean deviation; PSD: Pattern standard deviation; AGIS: Advanced glaucoma intervention score; IOP mean=33.56 mmHg, SD=3.40 mmHg
Sig 2 tailed test*, IOP: Intraocular pressure; MD: Mean deviation; PSD: Pattern standard deviation; AGIS: Advanced glaucoma intervention score; IOP mean=31.5 mmHg, SD=3.50 mmHg