The Oral Lichen Planus (OLP) is a chronic inflammatory disease which affects skin and oral mucosa [1]. OLP affects 1-2% of population and commonly occurs in adults over 40 years of age. It is a T-cell mediated autoimmune disease in which the cytotoxic CD8+ T cells trigger apoptosis of the basal cells of the oral epithelium [1]. C-reactive protein is an acute inflammatory protein that has been shown to increase up to 1000 fold at sites of infection or inflammation [2]. Salivary levels of immunoglobulins and acute phase proteins might have an important role to play in diagnosis of premalignant and malignant conditions [3]. Few studies have shown significant increase in salivary CRP in dysplastic OLP and oral squamous cell carcinoma [4].
Emotional stress has been associated with OLP for a long time. Erasmus Wilson (1869) categorised the patients with OLP as anxious, high strung and sensitive with a tendency of persistence and excessive worrying with periods of undue emotional distress [5]. Chaudhary S. et al., reported significantly higher levels of stress, anxiety and depression in patients with OLP when compared to healthy subjects [6].
A positive and significant correlation has been indicated between psychological distress, depression and CRP in a study done by Wium-Andersen MK, who examined 73131 individuals from the general population in Copenhagen [7].
However, a few studies were found in the literature which have reported alteration in salivary CRP levels in patients with OLP as compared to healthy individuals [3,9]. Also, considering that both altered salivary CRP levels and psychological stress have been reported in OLP patients [4,6], there are no studies which have reported the correlation between salivary CRP, OLP and psychological factors. Hence, it was hypothesised that there could be some alterations in salivary CRP levels in patients with OLP as compared to normal individuals. To the best of our knowledge, no such study has been reported so far and hence, a need was felt to conduct a study to assess the possible correlation between psychosocial factors and CRP levels.
Materials and Methods
This observational case-control study was undertaken in Department of Oral Medicine and Radiology, Sinhgad Dental College and Hospital, Pune, Maharashtra, India from August 2019 to January 2020. Sample size of 66 participants was correctly calculated using a formula given by Fliess [10]. Clearance certificate was obtained from the Scientific Advisory Committee of Institution (Ref.no:SDCH/SAC/2020-21/02).
Inclusion criteria: The age of participants ranged between 18-51 years. Participants were divided into two groups. Group A consisting 33 patients with clinically diagnosed OLP having bilateral lesions with white striae (according to Modified World Health Organisation (WHO) clinical diagnostic criteria of OLP and OLL 2003) [11] and group B with 33, age and gender matched healthy controls. Only the patients who gave consent were included in the study.
Exclusion criteria
Patients with systemic/inflammatory disease as per the data obtained from patient’s medical history medical history;
Patients on antibiotic/corticosteroid/NSAIDS therapy;
Lichenoid reactions to drug/restorations;
Chronic alcoholics were excluded to rule out the altered liver function that could be responsible for alteration in CRP levels.
The project was preceded by a pilot study. The participants from pilot study were also part of main study. Pilot study included 10 cases and 10 control group participants.
Data Collection
An informed consent was acquired from each participant after acquainting them with the study and providing patient information sheet. The patients were interviewed by a single calibrated researcher. Researcher was a postgraduate, who was trained for thorough clinical examination of OLP patients by senior Oral Medicine professional.
The demographic details and the history of the patient as well as the clinical findings were entered in a self- structured and peer reviewed proforma. The thorough clinical examination was also conducted by the same individual. Clinical appearance of the lesion; whether it was hypertrophic, erosive, ulcerative/bullous form was noted. This study consisted of 90% of patients with hypertrophic OLP and 10% were of erosive variant. For group A, analysis of pain/burning sensation was carried out using Visual Analogue Scale. Patients were asked to score from 0-to-10 with “0” representing the absence of pain/burning and “10” the worst pain sensation/burning ever [12].
Each participant in both the groups were given HADS questionnaire which consisted of 14 items [13]. All items in the questionnaire were explained to participants in their own language and the questionnaire was self-administered by them. They were asked to respond by scoring from zero to three according to severity of the symptoms. Scores between 8-10 were suggestive of borderline psychological morbidity and scores at and above 11 of a significant case of anxiety or depression.
Saliva Collection
Two mL of unstimulated saliva was collected by draining method in sterile container (Eppendorf tube). All the samples were collected at stable room temperature. Patients were instructed earlier not to take food or any other substance for two hours before saliva collection. All the samples were taken in the morning between 9 am-11am.
Laboratory methods
Saliva was centrifuged after collection at 3000rpm for 10 minutes and CRP levels were estimated using Mispa i2 Nephelometry [14].
Statistical Analysis
Data collected were sorted and categorised based on the parameters. Data were analysed using Unpaired t-test and Correlation analysis by Karl Pearson’s correlation coefficient using SPSS software version 21. The p-value <0.05 was considered significant in both analysis.
Results
Among both groups, 60% of study participants were females and 40% were males. In this study, the mean age was 37.80±9.18 years in group A and 35.50±7.42 years in group B. 90.9% of patients were of hypertrophic variant and 9.1% were of erosive variant of OLP [Table/Fig-1].
Distribution of case on the basis of tissue abuse habit and variant in OLP.
| Variables | No. of participants n (%) |
|---|
| Case tissue abuse habit | No habit | 26 (78.8) |
| Pan | 4 (12.1) |
| Tobacco | 3 (9.1) |
| Total | 33 (100) |
| Case variant in OLP | Hypertrophic | 30 (90.9) |
| Erosive | 3 (9.1) |
| Total | 33 (100) |
OLP: Oral lichen planus
Mean salivary CRP value among group A was 3.42±2.786 and among group B was 2.84±0.848 [Table/Fig-2]. Although the mean CRP was greater in group A, the difference was not statistically significant (p=0.537) [Table/Fig-3].
Mean and standard deviation of study variables.
| Variables | Minimum | Maximum | Mean | Std. Deviation |
|---|
| Case VAS score | 2.00 | 8.00 | 5.80 | 1.61933 |
| Case HADS score | 3.00 | 11.00 | 6.60 | 2.79682 |
| Case CRP value | 1.60 | 9.10 | 3.42 | 2.78640 |
| Control CRP value | 1.70 | 4.10 | 2.84 | 0.86879 |
| Control HADS | 1.00 | 4.00 | 2.70 | 0.94868 |
VAS: Visual analogue scale; HADS: Hospital anxiety and depression scale; CRP: C-reactive protein
Comparison of mean CRP and HADS value among cases and controls.
| Variables | Group | Mean | Std. Deviation | Mean Difference | t | p-value |
|---|
| CRP value (ng/mL) | Case | 3.4200 | 2.78640 | 0.58000 | 0.630 | 0.537 |
| Control | 2.8400 | 0.84879 |
| HADS score | Case | 6.6000 | 2.79682 | 3.90000 | 4.176 | 0.001* |
| Control | 2.7000 | 0.94868 |
HADS: Hospital anxiety and depression scale; CRP: C-reactive protein; Independent sample t-test
*p<0.05 statistically significant
Mean HADS score among group A was 6.60±2.796 and among group B was 2.70±0.948 [Table/Fig-2]. The mean difference was 3.900 and this difference was statistically significant (p=0.001) [Table/Fig-3]. No stressful event was reported in all participants from group B.
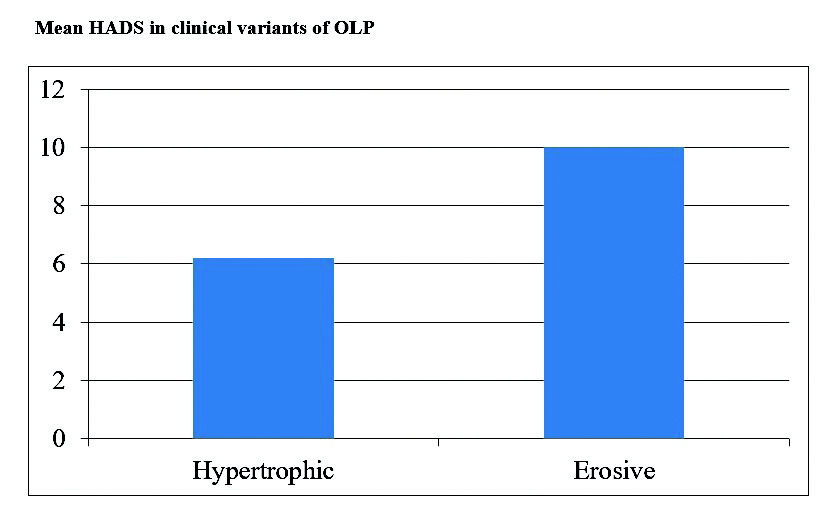
From group A, 20 (60.6%) participants were categorised normal, 10 (30.3%) were borderline while three participants (9.1%) were into morbid category [Table/Fig-4]. Among the clinical subtypes of OLP, Mean HADS was found to be greater in erosive OLP [Table/Fig-5]. Mean VAS among group A was 5.80±1.619 [Table/Fig-2]. A 60.6% of patients showed no or minimal pain/burning while 39.4% patients presented with moderate to severe burning.
Hospital anxiety and depression categories.
| Category | OLP n (%) | Controls n (%) |
|---|
| Normal | 20 (60.6) | 33 (100) |
| Borderline | 10 (30.3) | 0 |
| Morbid | 3 (9.1) | 0 |
Mean HADS in clinical variants of OLP.
No statistically significant correlation could be established between salivary CRP and HADS values (p=0.200) [Table/Fig-6].
Correlation of HADS, VAS and CRP value among cases.
| Scores of cases | HADS score | VAS score | CRP value |
|---|
| HADS score | Pearson Correlation (r) | 1 | 0.373 | 0.443 |
| p-value | | 0.289 | 0.200 |
| VAS score | Pearson Correlation (r) | 0.373 | 1 | -0.029 |
| p-value | 0.289 | | 0.938 |
| CRP value | Pearson Correlation (r) | 0.443 | -0.029 | 1 |
| p-value | 0.200 | 0.938 | |
VAS: Visual analogue scale; HADS: Hospital anxiety and depression scale; CRP: C-reactive protein; Pearson correlation test at 95% confidence interval. p<0.05 statistically significant
Discussion
Saliva is suggested to be a bio fluid that is assumed to be a mirror of the general health of the individual. It is shown to act as first line of defense against oxidative stress [8]. Considering its non invasive nature and it being relatively stress free alternative diagnostic tool in comparison with blood, Salivary CRP levels were incorporated in our study. It has been stated that the process of oxidative stress plays an important role in pathogenesis of OLP [15]. C-reactive protein is an acute phase protein and a marker of chronic inflammation which is synthesised in liver and has been identified as an important biomarker of wide spectrum of disease conditions such as systemic infection, rheumatoid arthritis, vasculitis. Adipocytes such as IL-6/TNF-alpha are closely related with CRP. Rhodus NL et al., reported greater levels of proinflammatory cytokines that is IL-6, IL-8, TNF-alpha in patients with ulcerative forms of OLP [16].
To establish the psychometric component in aetiopathogenesis of OLP, different instruments have been used. Questionnaires that are in practice are General Health Questionnaire-28, Cornell medical index, Social readjustment scale, Psychological Mineostta Multiphase, Speilberger State-Trait anxiety inventory [17].
In this study, a pre validated HAD scale was used which was developed by Zigmond AS and Snaith RP, and is established as a sensitive screening tool [13]. The results of the present study showed that there was significant increase in HAD scores in OLP patients compared to healthy controls. The results are in accordance with Araya S et al., and Sandhu SV who established a positive relationship between psychological alterations and OLP [5,17]. Various researchers have also investigated the role of psychological factors in relation with different variants of OLP [6,17]. Few revealed greater levels of anxiety in non erosive form of OLP [18], whereas some have associated it with erosive bullous OLP [19].
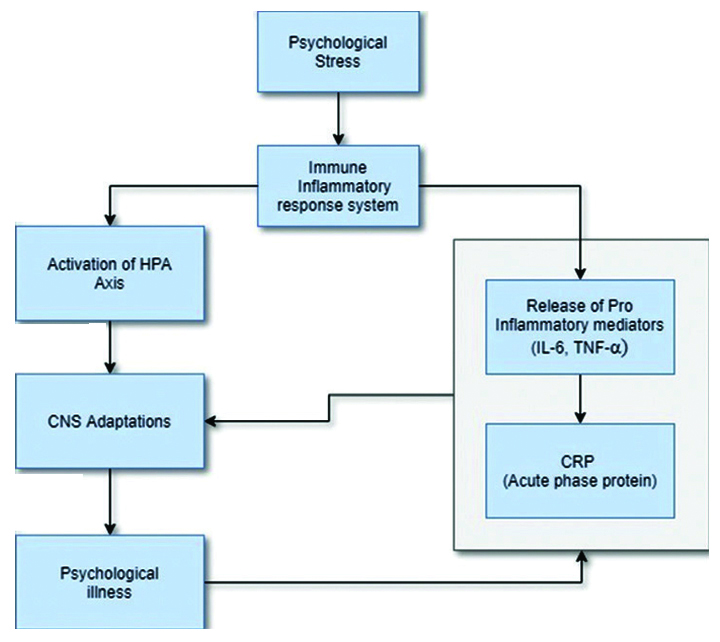
There have been reports stating greater stress and anxiety as well as increased salivary CRP in patients with OLP as compared to normal individuals [5,6,8,17,20] more so in erosive variant. In this study, greater levels of stress were seemed to be associated with erosive form of OLP. This could be attributed to pre-operative symptoms in erosive form of OLP which in itself can be a reason of stress. Although the chronic stress significantly affects CRP, the stress in itself does not cause any alteration in salivary CRP levels. There is still a controversy concerning the role of stress as a marker or causal factor in aetiopathogenesis of Lichen planus [Table/Fig-7] [22]. Similar observations were made in the present study.
Possible link between psychological stress and CRP [22].
Few authors have studied racial and gender differences in the distribution of CRP levels among black and white subjects. It has been found that women and black subjects have greater CRP levels compared to men and white subjects respectively [23]. In our study mean CRP, HADS and VAS scores were greater in participants of group A. However, no significant correlation could be found amongst them. Although, mean HADS was more in erosive variant, no such relation could be established between salivary CRP and clinical variants of OLP. Vankadara S et al., found greater levels of serum CRP in erosive OLP and speckled leukoplakia [24].
Results of the present study are comparable to studies done by few researchers in the past [Table/Fig-8,9] [5,6,8,9,17,18,20,21,24,25]. They found greater levels of salivary CRP in OLP and Burning Mouth Syndrome (BMS) patients compared to healthy subjects [8]. Metgud R and Bajaj S determined serum as well as salivary CRP in Oral Premalignant Diseases (OPMD) and oral squamous cell carcinoma [9]. He pointed out higher levels of mean CRP in OPMD than controls. Thus, it can be stated that CRP may be used as a diagnostic/prognostic marker in OLP.
Previous studies evaluating CRP in Oral Lichen Planus (OLP) patients [8,9,20,24,25].
| Author | CRP | Conditions | Conclusions |
|---|
| Kumar and Bhateja S (2011) [25] | Serum CRP | Oral Precancer [Leukoplakia, Oral submucous fibrosis (OSMF) & OLP] | Significant difference in mean CRP between case and control group |
| Metgud R and Bajaj S (2015) [9] | Salivary and serum CRP | Oral premalignant disorders (OSMF, OLP) and Oral squamous cell carcinoma | Mean CRP levels were higher in patients with oral premalignant lesions compared to controls. |
| Tvarijonaviciute A et al., (2017) [8] | Salivary CRP | OLP & Burning Mouth Syndrome (BMS) | Increased C-reactive protein in OLP patients compared to BMS and control patients |
| Vankadara S et al., (2018) [24] | Serum CRP | Oral Premalignancies and Malignancies | CRP level is a potential marker of increased risk of cancer |
| Shiva A et al., (2020) [20] | Salivary and serum CRP | OLP | CRP values were significantly higher both in serum and in saliva. CRP and nitric oxide may have a role in pathogenesis of OLP |
| Present study | Salivary CRP + Hospital Anxiety and Depression scale (HAD) | OLP | Difference in mean HADS was statistically significant in OLP compared to control group.No significant correlation was established between CRP and HADS in OLP (p=0.200) |
Previous studies evaluating stress/anxiety in Oral Lichen Planus (OLP) patients [5,6,17,18,21].
| Author | Questionnaire | Conditions | Conclusions |
|---|
| McCartan BE (1995) [18] | Cattell 16 PF Questionnaire.Hospital Anxiety and Depression (HAD) Scale | OLP (50 patients) | Anxiety scores elevated by 50%. |
| Soto Araya M et al., (2004) [5] | Test of Recent Experience (T.R.E.) Hospital Anxiety and Depression scale (HAD)
| OLP (9 patients) | OLP patients showed statistically significant differences for stress and anxiety in comparison with the control group (p<0.05).A positive relationship was establishedbetween psychological alterations and OLP. |
| Chaudhary S (2004) [6] | The General Health Questionnaire (GHQ) Hospital Anxiety and Depression Scale (HAD)
| OLP (41 patients) | Significantly higher stress, anxiety and depression levels were found in the OLP group.Psychological stressors play an important role in the causation of OLP. |
| Sandhu SV (2014) [17] | Hospital Anxiety and Depression Scale (HAD) | OLP (49 patients) | Definitive relationship between a stressful life event and onset and progression of OLP. |
| Kalkur C et al., (2015) [21] | DASS-42 questionnaire | OLP (25 patients) | OLP patients showed higher frequency of depression, anxiety and stress compared to control group.DASS-42 is a valid and consistent measurement tool of depression, anxiety, and stress. |
There have been reports showing significant difference in mean CRP levels between OPMD and control group [8,9,24,25]. In another study, after evaluation of serum and salivary CRP and nitric oxide, researchers observed significantly greater CRP levels in OLP patients compared to controls. They supported the potential use of salivary and serum CRP as a parameter for systemic levels of inflammation [20]. Very few studies were found where authors have reported comparable serum and salivary CRP levels [9,20]. Shiva A et al., found a significant correlation between serum CRP and nitric oxide value and saliva [20]. On the other hand, some studies found no significant difference in serum levels of CRP and other oxidative markers in patients with OLP before and after treatment [26].
The findings of the present study suggests that salivary CRP may be used as a non invasive and prognostic marker for OLP and it would be more helpful, if baseline values of CRP could be evaluated during treatment and follow-up visits of patients, which would further enhance its role in the clinical management of the disease. Also, psychological analysis and counselling should be made a part of the management protocol for patients with oral lesions like OLP and preferably for all the patients in Outpatient Department. There are alterations in salivary CRP levels in OLP patients. However, this alteration may not be correlated with the psychological factors.
Limitation(s)
The major limitations of the present study were small sample size and patients having tissue abuse habits.
Conclusion(s)
Increased salivary CRP levels in OLP patients compared to healthy individuals indicate that CRP could be used as a diagnostic marker in oral premalignant lesions. Psychological stressors in OLP patients should be identified early and counselling should be done in those patients. Further research in this direction is essential to ascertain the importance of stress counseling in the management protocol of OLP patients. No significant correlation between psychological status and CRP levels could be established in patients with OLP.
Further longitudinal studies with larger sample size are indicated to investigate the possible correlation between salivary CRP and HADS in patients with OLP. Role of salivary CRP as a diagnostic and prognostic marker for oral lesions need to be explored further to draw definitive conclusions. Also, the role of salivary CRP as a diagnostic and prognostic marker for oral lesions need to be explored to draw definitive conclusions.
OLP: Oral lichen planus
VAS: Visual analogue scale; HADS: Hospital anxiety and depression scale; CRP: C-reactive protein
HADS: Hospital anxiety and depression scale; CRP: C-reactive protein; Independent sample t-test
*p<0.05 statistically significant
VAS: Visual analogue scale; HADS: Hospital anxiety and depression scale; CRP: C-reactive protein; Pearson correlation test at 95% confidence interval. p<0.05 statistically significant