Coronavirus disease (COVID-19) is the worst pandemic in the past 100 years and has affected millions of people in more than 200 countries. The novel severe acute respiratory syndrome coronavirus (SARS-COV-2) was identified as the causative agent. A greater risk of COVID-19 has been reported in patients with diabetes and hypertension. Comparatively, the SARS epidemic in 2003, also caused by a beta coronavirus, was associated with a 3-fold risk of poor outcomes in the presence of diabetes, the highest among all comorbidities [1].
Diabetes mellitus, as a pre-existing health condition is a chronic low grade inflammation, which causes a dysregulated immune response, microvascular injury with a procoagulant state. A study of 191 patients reported a mortality risk of 2.85 fold for patients with diabetes [2]. In patients with diabetes the insulin resistance, poor glycaemic control leads to alveolar capillary microangiopathy and interstitial fibrosis via over inflammation. A systematic review by Huang I et al. revealed that diabetes was associated with mortality, severity, acute respiratory distress syndrome, and disease progression in patients with COVID-19 [3]. In COVID-19, dichotomous outcomes of disease severity such as ICU admission and mortality were significantly elevated (~2- and ~3-fold, respectively) in diabetes. This is of particular interest because Huang I et al., noted an increased risk of a composite poor outcome in patients with diabetes, which included severe COVID-19 as one of the outcomes. One study of 1382 COVID-19 patients with diabetes found a 2.79-fold risk of admission to the ICU [4].
The present study aimed to find the association between diabetes as a co-morbidity and their pre-existing glycaemic control with clinical course of disease in COVID-19 patients and their oxygen requirement during the treatment.
Materials and Methods
This single centre retrospective observational study was conducted from June 2020 to September 2020 on (51 diabetic and 51 non diabetic) patients, at Kamineni Academy of Medical Sciences, Hyderabad, India. The record of the patients diagnosed with COVID-19 which includes diabetic and non diabetic patients and data, including age, sex, history of diabetes, HbA1c, level of illness severity (oxygen requirement) were noted.
Inclusion criteria: Data of Real Time-Reverse Transcription Polymerase Chain Reaction (RT-PCR) positive patients, with type-II diabetes mellitus and non diabetics of age group >30 years was included in the study.
Exclusion criteria: COVID-19 RT-PCR negative patients data was excluded from the study.
All the subjects in the complete article were called Diabetics and non diabetics according to their history of Diabetes. also the HbA1c of all subjects mentioned and tabulated in results gave us a history of glycaemic control for past three months for all of them.
Statistical Analysis
Data were entered into a Microsoft Excel datasheet and were analysed using SPSS version 22.0 (IBM SPSS Statistics, Somers NY, USA) software. Categorical data was represented in the form of frequencies and proportions in MS Excel and MS word. Chi-square test was used as a test of significance for qualitative data and p-value <0.05 was considered as statistically significant.
Results
Among diabetics, the majority of subjects, 16 (31.4%) were in the age group 50 to 59 years and among non diabetics majority, 14 (27.5%) were in the age group 40 to 49 years. There was no significant difference in age distribution between diabetics and non diabetics [Table/Fig-1].
Profile of subjects in the study.
| Demographic variables | Diabetes | p-value |
|---|
| Yes | No | Total |
|---|
| Count | % | Count | % | Count | % |
|---|
| Age (years) | 30-39 | 4 | 7.8% | 12 | 23.5% | 16 | 15.7% | 0.053 |
| 40-49 | 9 | 17.7% | 14 | 27.5% | 23 | 22.7% |
| 50-59 | 16 | 31.4% | 13 | 25.5% | 29 | 28.4% |
| 60-69 | 7 | 13.7% | 6 | 11.7% | 13 | 12.7% |
| >70 | 15 | 29.4% | 6 | 11.8% | 21 | 20.6% |
| Sex | Male | 39 | 76.5% | 37 | 72.5% | 76 | 74.5% | 0.650 |
| Female | 12 | 23.5% | 14 | 27.5% | 26 | 25.5% |
Among diabetics, 39 (76.5%) were males and 12 (23.5%) were females and among non diabetics, 37 (72.5%) were males and 14 (27.5%) were females. There was no significant difference in sex distribution between diabetics and non diabetics patients [Table/Fig-1].
Among diabetics, 4 (7.8%) had normal HbA1c, 9 (17.6%) had Pre-diabetic range and 38 (74.5%) had Diabetic Range >6.5% [Table/Fig-2].
HbA1c distribution among diabetics.
| HbA1c measurements | Diabetic patients |
|---|
| Count (n) | Percentage (%) |
|---|
| Normal <5.7% | 4 | 7.8% |
| Prediabetic range 5.7%-6.5% | 9 | 17.6% |
| Diabetic range >6.5% | 38 | 74.5% |
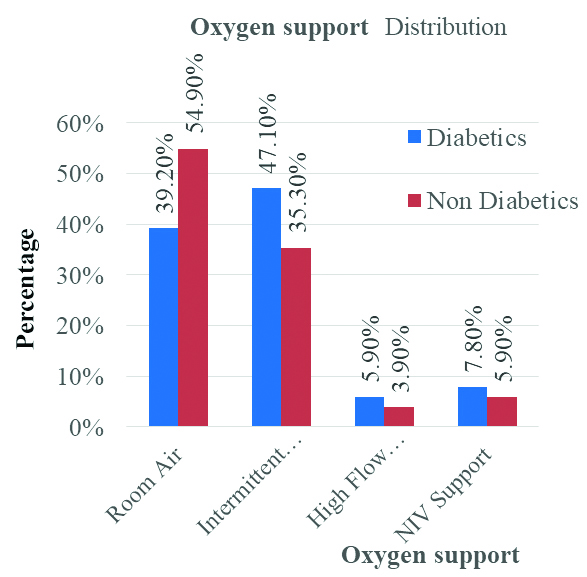
In the study among diabetics, 20 (39.2%) required room air, 24 (47.1%) intermittent oxygen, 3 (5.9%) high flow oxygen, and 4 (7.8%) NIV support. Among non diabetics, 28 (54.9%) required room air, 18 (35.3%) intermittent oxygen, 2 (3.9%) high flow oxygen, and 3 (5.9%) NIV support. There was no significant difference in oxygen support between diabetics and non diabetics. (p-value: 0.469) [Table/Fig-3,4].
Oxygen support comparison between diabetics and non diabetic patients (χ2=2.53, differential factor=3, p=0.469).
| Oxygen support data of subjects | Diabetes |
|---|
| Yes | No | Total |
|---|
| Count (n) | Percentage (%) | Count (n) | Percentage (%) | Count (n) | Percentage (%) |
|---|
| Room air | 20 | 39.2% | 28 | 54.9% | 48 | 47.1% |
| Intermittent oxygen | 24 | 47.1% | 18 | 35.3% | 42 | 41.2% |
| High flow oxygen | 3 | 5.9% | 2 | 3.9% | 5 | 4.9% |
| NIV support | 4 | 7.8% | 3 | 5.9% | 7 | 6.9% |
| Total | 51 | 100.0% | 51 | 100.0% | 102 | 100.0% |
Bar diagram showing Oxygen support comparison between diabetics and non diabetics patients.
Discussion
The prevalence of diabetes mellitus is anticipated to increase substantially during the next decades worldwide and considered to be the main cause of human deaths [5]. People with diabetes are more susceptible to certain infectious diseases, such as Staphylococcus aureus and Mycobacterium tuberculosis, possibly because of their dysregulated immune system [6,7]. Hyperglycaemia can impair host defences and poor glycaemic control has been associated with infections. Given that glycaemic control is a modifiable factor and can be achieved and sustained by health care interventions.
It is known clearly that the interplay between COVID-19 and diabetes mellitus entails complex pathophysiology The surface proteins of the SARS-CoV-2 virus could attack heme on the 1-β chain of haemoglobin in red blood cells of individuals with diabetes, dissociating iron to form porphyrin, thereby causing less and less haemoglobin that can carry oxygen, and carbon dioxide ultimately leading to symptoms of respiratory distress [8-10]. It is suggested that de-oxy haemoglobin is more vulnerable than oxidised haemoglobin to the surface proteins of the SARS-CoV-2 virus, and glycosylated haemoglobin (deoxygenated form) levels are higher in diabetic patients [8]. These findings suggest that patients with hyperglycaemia or diabetes may be more vulnerable to SARS-CoV-2 attack due to enhanced glycation of haemoglobin, thus increasing the risk for COVID-19 associated mortality rate.
The pathogenic mechanism of how the SARS-CoV-2 virus binds to the 1-β chain of porphyrins of the erythrocytes, leading to release of iron and disturbance of heme-metabolism, remains elusive and needs further investigation.
Diabetes is well known to induce pulmonary dysfunction, such as reducing lung volumes and compliance and increasing airway resistance, which is related to insulin resistance and nonenzymatic glycosylation of lung proteins [11]. This may be another risk factor for a more-severe illness in COVID-19, the SARS-CoV-2 infection could cause hyper-inflammation in cells, resulting in excessive activation of macrophages, which can suppress the recruitment of T cells [12]. Emerging evidence shows that during the acute phase of infection, SARS-CoV-2 invades CD4+ T and CD8+ T lymphocytes, leading to cell apoptosis and lymphocytopenia [13]. It has been shown that elevated glucose levels promote SARS-CoV-2 replication in monocytes, resulting in inhibition of T cell response [14] diabetes is well known to be associated with dysfunctional innate and adaptive immunity [15] and it is thus speculated that impaired immune function in diabetes patients may accentuate SARS-CoV-2 infection and its harmful function.
Recent researches revealed that cytokine storm emerges in COVID-19 patients with hyperglycaemia or Type 2 Diabetes Mellitus (T2DM), leading to dysregulation of immune response and Acute Respiratory Distress Syndrome (ARDS) [16].
Increased levels of chronic inflammatory factors, such as IL-1β, IL-6, TNF-α, and activation of the immune response are frequently found in diabetic patients with poor-controlled blood glucose levels [17]. Thus, high blood glucose may promote cytokine storm emerge and immune over-activation, ultimately causing ARDS and multiple organ failure in COVID-19 patients. It may therefore be more appropriate to say that complications and co-morbidities linked to diabetes are associated with a higher mortality rate than the presence of diabetes per se [18,19].
Limitation(s)
The major limitations of the present study was the small sample size and the interpretation of the statistical significance of the mentioned data require a lot of caution and skill.
Conclusion(s)
The outbreak of the COVID-19 pandemic has caused a global health crisis of our time. A growing body of clinical evidence shows that patients with pre-existing diabetes are highly susceptible to SARS-CoV-2 infection and its associated mortality. The exact mechanisms linking diabetes and COVID-19 remain to be further elucidated, but our observations suggest that poor glycaemic control may play critical role in the severity of COVID-19 in patients with pre-existing diabetes and showed higher oxygen requirement as compared to diabetics with good glycaemic control. The requirement for further studies involving a larger sample size needs to be considered to draw further conclusions.