Postoperative pain is inevitable. Inadequate pain control or analgesia leads to numerous biochemical and physiological stress responses which may lead to delayed recovery and prolonged hospital stay irrespective of age. In paediatric age group, the dermatomal nerve endings for nociception are more denser in comparison to adults and assessment of pain is difficult so a good and durable perioperative analgesic is imperative in them [1].
Caudal block is one of the most routinely performed, easier, safe, reliable and efficient regional anaesthetic technique for pain management in paediatric infra-umbilical surgeries for both intraoperative and postoperative analgesia. The main disadvantage of single shot caudal block is that it provides shorter duration of analgesia which increases the requirement for analgesic supplementation postoperatively [2].
Innumerable drugs have been used as adjuncts to local anaesthetics in caudal block to enhance the efficacy of postoperative analgesia like opioids, α2-agonists, neostigmine, epinephrine, ketamine etc. However, the use of caudal and systemic opioids produces various side-effects including nausea, vomiting, pruritus, urinary retention, or respiratory depression. Similarly, epidural administration of α2-agonists like dexmedetomidine and clonidine may cause profound hypotension, bradycardia, and sedation [3,4]. The addition of epinephrine with local anaesthesia causes hypertension and tachycardia [5]. Ketamine may lead to neurotoxicity on accidental intrathecal injection.
Ropivacaine is a relatively new amide-type long acting pure S-enantiomer local anaesthetic. It is preferred for paediatric caudal anaesthesia and analgesia as it produces lesser motor blockade and displays lower cardiovascular and central nervous system toxicity than bupivacaine [6]. Dexamethasone is one of the popular drugs long established to decrease nausea, vomiting and pain perioperatively with the overall goal to ensure a better and smooth recovery [7]. Furthermore, several studies have demonstrated that addition of dexamethasone in caudal block reduces pain and analgesic requirement in postoperative period [8].
Very few studies have been conducted to assess the efficacy of dexamethasone as an adjunct to local anaesthetic particularly ropivacaine. Recent studies have reported that dexamethasone in its lower doses (0.1 mg/kg) used as an adjuvant to ropivacaine provides effective and prolonged postoperative analgesia by overcoming the disadvantages of single shot caudal epidural with local anaesthetic alone which could be beneficial in clinical paediatric anaesthesia practice in terms of its efficacy and safety as the higher doses (0.2 mg/kg or more) may have unfavourable side-effect profile [2,9,10].
The present study was based on the hypothesis that addition of dexamethasone to ropivacaine (0.2%) in caudal block would potentiate the effect of ropivacaine and prolong the duration of analgesia with minimal adverse effects. So, this study was conducted to evaluate the efficacy and safety of addition of dexamethasone (0.1 mg/kg) to ropivacaine 0.2% in caudal block for postoperative analgesia in paediatric patients posted for infra-umbilical surgeries. The duration of postoperative analgesia was the primary objective while rescue analgesic consumption (when FLACC score ≥4), intraoperative haemodynamic changes and postoperative side-effects were secondary objectives of this study.
Materials and Methods
This randomised double-blinded controlled study was conducted on 80 paediatric patients at a tertiary care Teaching Institute in Rajasthan, India after obtaining approval (No.2370/Acad-III/MCA 2016 dated 18/12/2018) from Institutional Ethical Committee from April 2019 to September 2019.
Inclusion criteria: After obtaining written and informed consent from parents, children in the age group of 8 months to 8 years belonging to American Society of Anaesthesiologists (ASA) physical status I or II posted for various infra-umbilical surgeries during the study time period were included in this study.
Exclusion criteria: The children whose parent’s refused to give consent, those mentally retarded with delayed developmental milestones or neurological diseases, ones with suspected coagulopathy, known hypersensitivity to the study drugs, infection and any skeletal deformities in the caudal area were excluded from the study.
Sample size calculation: The sample size was calculated based on an observation made by a previous study [3]. The sample size was calculated to be 40 patients in each group by assuming a detection of mean difference of 2 with an expected standard deviation of 2.45 between the two groups in terms of time of requirement of first rescue analgesic with an alpha error of 0.01 and 90% power.
Patients were randomly allocated into two groups with 40 patients in each group: Group R (control group, ropivacaine alone; n=40) and Group RD (ropivacaine with dexamethasone; n=40) using a computer-generated table of random numbers. The sequentially numbered opaque sealed envelope method was adopted for allocation concealment. Patients in Group R were administered 0.2% ropivacaine 1 mL/kg+1 mL Normal Saline (NS) while patients in Group RD were administered 0.2% ropivacaine 1 mL/kg+dexamethasone 0.1 mg/kg (diluted in 1 mL NS) in caudal block [Table/Fig-1].
For the purpose of double blinding, the study drugs were prepared and administered by a resident anaesthesiologist who was not involved in the study, while the another anaesthesiologist observed the patients thereafter who was unaware about the contents of syringes.
Study Procedure
All the children underwent a detailed preoperative assessment the day before surgery and were kept nil per oral as per standard fasting guidelines for paediatric patients prior to surgery. The ASA standard monitoring, which included Heart Rate (HR), Non Invasive Blood Pressure (NIBP), Electrocardiogram (ECG), Oxygen Saturation (SpO2) and temperature of patients in operating room, was attached. After securing Intravenous (IV) access, ringer lactate was started, midazolam 0.05 mg/kg IV and glycopyrrolate 0.004 mg/kg IV were administered as premedication. After pre-oxygenation with 100% O2 for 3 minutes, patients were induced with either propofol 2-3 mg/kg IV or sevoflurane (8%) in 50:50 Nitrous Oxide (N2O) and oxygen (O2). Airway was secured with appropriate sized endotracheal tube using atracurium 0.5 mg/kg IV as loading dose and anaesthesia was maintained with O2/N2O (50:50), sevoflurane (1-2%) and atracurium 0.1 mg/kg IV as maintenance doses along with controlled ventilation.
Under strict aseptic technique, caudal block was performed in lateral decubitus position. Under all aseptic precautions, caudal block was performed after proper identification of anatomical landmarks (two posterior superior iliac spines and sacral hiatus) with the help of a 22 gauge short bevelled needle. Following careful negative aspiration, drugs were administered according to the group allocation. After performing the block, the patients were turned back to supine position, and surgery allowed to proceed after ten minutes with continuous intraoperative monitoring of SpO2, HR, NIBP and Respiratory Rate (RR) every five minutes. At the time of surgical incision, absence of rise in HR or Mean Arterial Pressure (MAP) of >20% from baseline was defined as adequate analgesia. The children in whom the caudal block could not be performed or inadequate analgesia (rise in HR or MAP >20%) at the time of surgical incision were excluded from the study.
During emergence from anaesthesia, once surgery was completed, patients were extubated following successful reversal with IV glycopyrrolate 0.008 mg/kg and IV neostigmine 0.5 mg/kg. In the post anaesthesia care unit or postoperative ward, haemodynamic parameters (HR, MAP, SpO2) were monitored till 24 hours after caudal block, initially hourly up to 8 hours followed by 2 hourly thereafter up to 24 hours. A paediatric observational FLACC [11] scale (0-10 score) was used to assess pain. The FLACC score were graded as (0=no pain; 1-3=mild pain; 4-7=moderate pain; 8-10=severe pain), and it was also assessed every hour up to 8 hours, then 2 hourly till 24 hours after caudal block [Table/Fig-2]. Rescue analgesic (oral paracetamol 15 mg/kg) was administered when FLACC ≥4. The rescue analgesic consumption in terms of number of doses required in 24 hours and duration of analgesia i.e. from the time of administration of caudal block to the time when FLACC ≥4 were noted.
| Categories | 0 | 1 | 2 |
|---|
| Face | No particular expression or smile | Occasional grimace or frown; withdrawn, disinterested | Frequent to constant frown, clenched jaw, quivering chin |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking or legs drawn up |
| Activity | Lying quietly, normal position, moves easily | Squirming, shifting back and forth, tense | Arched, rigid, or jerking |
| Cry | No cry (awake or asleep) | Moans or whimpers, occasional complaint | Crying steadily, screams or sobs; frequent complaints |
| Consolability | Content, relaxed | Reassured by occasional touching, hugging, or being talked to; distractable | Difficult to console or comfort |
*FLACC: Face legs activity cry and consolability
Various side effects or complications like hypotension, bradycardia, PONV, respiratory depression, postoperative agitation etc. were also noted. Ondansetron (0.1 mg/kg) was given to manage PONV. A fall in systolic blood pressure i.e. SBP <70 mmHg plus twice the age in years along with altered peripheral perfusion was considered as hypotension while the HR <80 beats/min and <60 beats/min for children <1 year and >1 year respectively were considered as bradycardia. A rise in HR and/or MAP >20% of their baseline values was considered as failed caudal block.
Statistical Analysis
All the numerical and categorical data were presented as mean±SD (Standard Deviation) and numbers or frequency (percentage). The SPSS (Statistical Package for the Social Sciences) software version 16.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. The data of both the groups were compared using various qualitative and quantitative tests like unpaired and paired student t-test, Chi-square test etc. The p-value <0.05 were considered as statistically significant.
Results
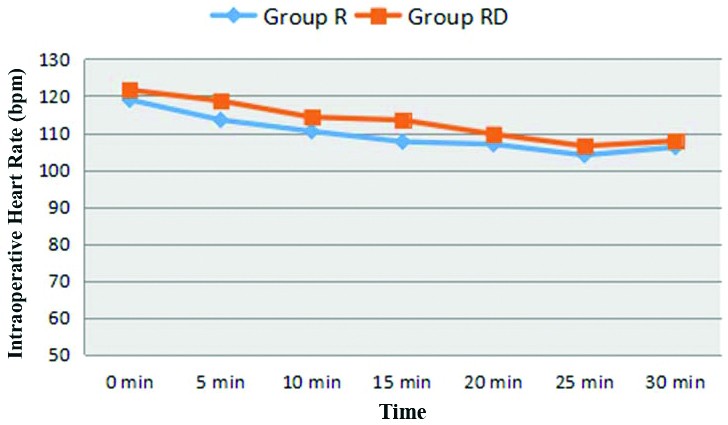
Demographic profile i.e., mean age, weight, gender, ASA physical status classification and duration of surgery were found to be comparable in two groups; (p-value >0.05) [Table/Fig-3]. Both the groups were also comparable in terms of baseline haemodynamic parameters. The mean SpO2 at baseline and thereafter at all time intervals was comparable in two groups (p-value >0.05). The difference in HR, SBP, DBP and MAP in two groups at various time intervals was found to be statistically insignificant (p-value >0.05); [Table/Fig-4,5].
Demographic profile and duration of surgery in two groups.
| Parameters | Group R (n=40) | Group RD (n=40) | p-value (Chi-square and student t-test) |
|---|
| Age (in months) | 41.38±22.69 | 39.83±21.98 | 1.00 |
| Gender (M/F) | 35/5 | 33/7 | 0.754 |
| Weight (kg) | 12.68±5.05 | 12.50±4.68 | 0.520 |
| ASA (I/II) | 32/8 | 34/6 | 0.558 |
| Duration of surgery (min) | 42.23±19.86 | 43.88±11.57 | 0.218 |
| Types of surgery |
| Herniotomy | 20 | 24 | |
| Orchidopexy | 11 | 9 | |
| Urethroplasty | 4 | 4 | |
| Others | 5 | 3 | |
Group R: Ropivacaine; Group RD: Ropivacaine+Dexamethasone; Values are expressed as mean±SD or number; F: Female; M: Male; ASA: American society of anaesthesiologists
Comparison of intraoperative HR in two groups.
Group R: Ropivacaine; Group RD: Ropivacaine+Dexamethasone; bpm: Beats per minute
Comparison of intraoperative haemodynamics (SBP, DBP and MAP) in two groups.
Group R: Ropivacaine; Group RD: Ropivacaine+Dexamethasone; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; MAP: Mean arterial pressure

The FLACC pain scores were found to be higher in Group R as compared to Group RD. None of the patients had FLACC ≥4 during initial 4 hours in both of the groups. After that, at all time intervals FLACC pain scores were lower in Group RD compared to Group R which were statistically significant at 6th, 12th and 18th hour [Table/Fig-6].
Incidence of FLACC pain score ≥4 at various time intervals in two groups.
| Time interval (hours) | Incidence of pain score (FLACC) >4 in Group R (n=40) | Incidence of pain score (FLACC) >4 in Group RD (n=40) | p-value (Chi-square test) |
|---|
| 1 | 0 | 0 | - |
| 2 | 0 | 0 | - |
| 3 | 0 | 0 | - |
| 4 | 0 | 0 | - |
| 5 | 3 | 0 | 0.239 |
| 6 | 9 | 0 | 0.005 |
| 7 | 8 | 2 | 0.091 |
| 8 | 4 | 0 | 0.124 |
| 10 | 3 | 0 | 0.239 |
| 12 | 10 | 2 | 0.03 |
| 14 | 11 | 19 | 0.106 |
| 16 | 3 | 1 | 0.608 |
| 18 | 7 | 0 | 0.011 |
| 20 | 10 | 6 | 0.402 |
| 22 | 5 | 3 | 0.709 |
| 24 | 2 | 0 | 0.474 |
Group R: Ropivacaine; Group RD: Ropivacaine+Dexamethasone; FLACC: Face legs activity cry and consolability; bold p-values are significant
The duration of analgesia was found to be significantly longer in Group RD (745.21±146.91 min) compared to Group R (440.38±76.44 min) (p-value <0.001). The rescue analgesic consumption was significantly lower in terms of requirement of lesser number of doses of rescue analgesic in Group RD compared to Group R (p-value <0.05). So, the patients in Group R required more rescue analgesic as compared to Group RD [Table/Fig-7].
Comparison of duration of analgesia and rescue analgesic requirement in two groups.
| Parameters | Group R (n=40) | Group RD (n=40) | p-value (Student t-test) |
|---|
| Duration of analgesia (min) | 440.38±76.44 | 745.21±146.91 | <0.001 |
| Number of doses of rescue analgesic in 1st 24 hour n (%) |
| 1 | 15 (37.5%) | 8 (20%) | - |
| 2 | 8 (20%) | 3 (7.5%) |
| 3 | 10 (25%) | 0 (0%) |
Group R: Ropivacaine; Group RD: Ropivacaine+Dexamethasone; Values are expressed as Mean±SD and number (percentage)
Considering postoperative complications, fever was observed in 1 patient (3.33%) and PONV in 2 patients (6.66%) in Group R. None of the patients had similar/any complaints in Group RD. There were no incidences of bradycardia, hypotension, respiratory depression, and pruritus in both groups (p-value >0.05).
Discussion
The development of pain assessment tools specific to children has resulted in great progress in pain management of paediatric patients. In recent years, the approach for postoperative pain management in the paediatric patients has significantly changed. Caudal epidural block is one of the most popular regional anaesthetic and analgesic techniques used for postoperative analgesia in paediatric patients as it allows rapid recovery from anaesthesia with efficient postoperative analgesia. Dexamethasone as an adjuvant with local anaesthetic prolonged the duration of the caudal block, enhanced analgesia along with its opioid-sparing and antiemetic effect in the postoperative period [12-14].
Several studies have been conducted on dexamethasone, as an adjuvant to local anaesthetic in caudal block which have proved its analgesic efficacy in postoperative pain management in paediatric patients [2-4,8-10]. In recent years, perioperative use of dexamethasone has increased, especially in central and peripheral nerve blocks [15]. Unlike other adjuvants dexamethasone is not reported to be associated with any major side-effect in postoperative period [3]. So, caudal block has been chosen as modality of analgesia using 0.2% ropivacaine (1 mL/kg) with or without dexamethasone (0.1 mg/kg) in paediatric patients for this study.
Wulf H et al., studied the pharmacokinetics of ropivacaine 0.2% in paediatric age group and they documented that this particular dose of ropivacaine is safe in caudal block [16]. Ropivacaine is less cardiotoxic and produces less motor blockade as compared to bupivacaine, so ropivacaine was preferred for use as the local anaesthetic in this study. Stewart DW et al., found that 90% of patients undergoing orchidopexy without caudal block experienced substantial pain with requirement of postoperative analgesia which proved the necessity and importance of caudal block in paediatric patients [17].
In present study, the mean duration of analgesia was found to be significantly prolonged in Group RD (745.21±146.91 min) as compared to Group R (440.38±76.44 min). Yousef GT et al., used 0.15% ropivacaine 1.5 mL/kg with 0.1 mg/kg dexamethasone in caudal block and found significantly longer duration of analgesia in dexamethasone group (730±260 min) as compared to plain ropivacaine group (260±65 min) which coincides with the present results [18]. Similarly, Dongare DH and Karhade SS, also reported a considerably longer duration of analgesia in caudal dexamethasone group (626.33±59.39 min) as compared to IV dexamethasone group (194.67±27.76 min) [19]. They had used 0.25% bupivacaine 1.25 mL/kg as local anaesthetic and 0.1 mg/kg dexamethasone as an adjuvant through caudal route. These results also correlate to this study while difference in mean duration of analgesia can be explained due to use of bupivacaine as the anaesthetic in their study. Sridhar RB et al., also observed significantly prolonged duration of analgesia in ropivacaine with dexamethasone group (450.0±72.6 min) as compared to ropivacaine alone group (285.9±52.7 min) [10]. The difference in duration of analgesia may be due to lower dose i.e., 0.5 mL/kg 0.2% ropivacaine used in their study. Girgis K, noticed a protracted period of analgesia in bupivacaine (0.25%, 1 mL/kg) with dexamethasone (0.2 mg/kg) group (11.2±3.5 h) in comparison to plain bupivacaine (0.25%, 1 mL/kg) group (7.1±3.2h) [20]. This conspicuous lengthening in duration of analgesia might be attributed to higher dose of dexamethasone (0.2 mg/kg). Although researchers have used different concentrations of both/either bupivacaine and ropivacaine; nevertheless addition of dexamethasone (0.1-0.2 mg/kg) with local anaesthetic in caudal blocks has proved beneficial in extending the duration of analgesia mainly attributed to its prostaglandin reduction activity responsible for enhanced anti-nociception in inflamed tissues (anti-inflammatory property).
The postoperative pain scores (FLACC scale) were found to be analogous in both groups up to four hour as the approximate duration of action of ropivacaine is reported to be 4-6 hour [21], which could be the reason of insignificant difference in FLACC pain scores up to four hour in the present study. After that, at all time intervals FLACC pain scores were significantly lower in dexamethasone group as compared to ropivacaine alone group at 6, 12 and 18 hour. These results are consistent with the findings of Kim EM et al., who found significantly lower FLACC score in dexamethasone (0.1 mg/kg) group as compared to ropivacaine (0.15%, 1.5 mL/kg) alone group at 6 and 24 hour [3]. Parameswari A et al., found that the mean pain scores were similar in both bupivacaine (0.125%, 1 mL/kg) and bupivacaine with dexamethasone (0.1 mg/kg) groups for the first 4 hour, after that it was significantly lower in dexamethasone group at 5, 6, 16, 20, and 24 hour which concurs with the results of our study [22].
The rescue analgesic consumption was significantly lower in dexamethasone group as compared to ropivacaine alone group. In Group R, a majority of patients (33 patients) required rescue analgesic while in Group RD only 11 patients required rescue analgesic in first 24 hours. In addition, none of the patients in dexamethasone group required >2/3 doses of rescue analgesic. Kim EM et al., found that there were more number of patients in dexamethasone group (19 of 38;50%), who required no rescue analgesic postoperatively up to 48 h than in ropivacaine alone group (4 of 37;10.8%); (p-value <0.001); (p-value <0.001) [3]. Parameswari A et al., reported that 27 patients (41.5%) in dexamethasone group did not receive any rescue analgesic in 24 hour whereas all the patients in control group (bupivacaine) received at least one rescue analgesic dose and 62 patients (94%) received two or more number of rescue analgesic, which is substantially higher rivalled to dexamethasone group [22]. Parallely, Girgis K, found that the number of oral paracetamol doses required in the first 24 hour were radically lesser in bupivacaine with dexamethasone group compared to bupivacaine alone [20].
Dexamethasone has direct membrane stabilising action on nerves which may have a local anaesthetic effect. Dexamethasone regulates nuclear factor (NF)-kB; commonly associated in development of pathological pain, thus proving hyperalgesia can be inhibited by reduction in NF-kB levels which can be achieved by administration of epidural or caudal corticosteroid like dexamethasone [3]. Furthermore, dexamethasone might prevent postoperative central sensitisation for pain and augments the postoperative analgesia of caudal block. This mechanism of prevention of hyperalgesia at spinal cord level might be the reason for prolonged duration of analgesia along with lower pain scores and reduced rescue analgesic requirement in postoperative period in patients who received dexamethasone as an adjuvant with local anaesthetic in caudal block.
In the present study, HR and blood pressure (SBP, DBP and MAP) of all the patients were monitored at regular intervals and no significant haemodynamic changes were noted in any of the two groups which showed that patients remained haemodynamically stable throughout the perioperative period which depicted the favourable safety profile of dexamethasone. Sridhar RB et al., and Mohamed AZ, also found no significant haemodyamic changes in their respective studies [10,23].
As far as complications or adverse effects secondary to procedure and of adjuvant are concerned, none of the patients in any group had any specific complications apart from some minor side-effects like fever and PONV in patients in ropivacaine alone group. Dexamethasone is known to have antiemetic property which might prevent any PONV. None of the patients had bradycardia, hypotension, respiratory depression, and pruritus in both the groups.
However, higher doses of dexamethasone (0.2 mg/kg or more) may be associated with complications like increased tendency of postoperative wound infection, bleeding, transient adrenocortical suppression or hyperglycaemia [3] but in present study, the lower dose (0.1 mg/kg) of dexamethasone has been used which was not associated with any side-effect therefore favouring the use of dexamethasone (0.1 mg/kg) as a safe and effective adjuvant in caudal block.
Limitation(s)
The authors could not evaluate motor block, as it is unlikely at the 0.2% concentration of ropivacaine to develop significant motor blockade.
Conclusion(s)
The present study concludes that dexamethasone as an adjuvant leads to improved quality and duration of analgesia, decreased rescue analgesic requirement, stable haemodynamics and minimal or no side-effects. Dexamethasone (0.1 mg/kg) can be used as a safe and effective adjuvant to 0.2% ropivacaine in single shot caudal block for postoperative analgesia in paediatric patients undergoing various infra-umbilical surgeries.
Group R: Ropivacaine; Group RD: Ropivacaine+Dexamethasone; Values are expressed as mean±SD or number; F: Female; M: Male; ASA: American society of anaesthesiologists
Group R: Ropivacaine; Group RD: Ropivacaine+Dexamethasone; FLACC: Face legs activity cry and consolability; bold p-values are significant
Group R: Ropivacaine; Group RD: Ropivacaine+Dexamethasone; Values are expressed as Mean±SD and number (percentage)