Breast cancer is the most common cancer in women globally and with an incidence of one in four of all cancers. In India the age adjusted incidence rate and mortality rates are 25.8 per 100,000 and 12.7 per 100,000 women respectively. The incidence of breast cancer in the young and presentation at advanced stage is increasing in India compared to western population [1].
The NACT is currently established as a standard therapeutic approach for patients with locally advanced breast cancer [2-4]. Studies failed to show significant gain in survival benefit from NACT for breast cancer; however it has been shown to have certain advantages over other modalities. The efficacy of drug can be assessed in a relatively shorter time which helps in the modification of therapy in case of a poor response. The NACT downstages the tumours and renders the inoperable tumours resectable, thereby increasing the rate of breast-conservation surgery. The degree of response to NACT is also considered as a prognostic factor. Evaluation of gene expression profiles of tumour before, during and after treatment facilitates development of novel therapeutic agents [2-4].
The most important parameter to predict treatment success and overall survival is the achievement of a pCR. The pCR can only be determined by post NACT histopathological examination of primary site and nodes and is defined as absence of invasive cancer in breast or lymph node tissue. Presence of lymphovascular emboli at primary site or Isolated Tumour Cells (ITC) only in the lymph node in the absence of residual invasive carcinoma excludes pCR. However, presence of Ductal Carcinoma In Situ (DCIS) in the absence of residual invasive carcinoma is compatible with pCR [5].
The volume of residual disease can be subtle on gross and microscopy leading to considerable variability both in terms of pathology sampling and interpretation of response as highlighted in previous studies [6]. Many systems have been proposed to classify the degree of tumour response to therapy [7]. The RCB is an online tool for the quantification of residual disease that is developed by MD Anderson cancer hospital. It is easy to practice, reproducible, and the RCB score has been clinically validated as independent prognostic factor for long-term survival [8-10]. The RCB provides a standardised operating procedure for the evaluation of post NACT specimens, requiring only standard pathology materials with no additional cost [7,11].
The objective of this study was to evaluate the methodology for grossing and microscopic examination for assessing pathologic response in post NACT breast cancer specimens using RCB system developed by MD Anderson Cancer Institute [8] and its feasibility for routine practice in an average Indian laboratory setting.
Materials and Methods
The present study was a cross-sectional study conducted over a duration of three months from May 2018 to July 2018 after obtaining approval from Institutional Ethics Committee (IEC/2018/139.2). Written Informed consent was taken from all the patients.
Inclusion and Exclusion criteria: Patients with newly diagnosed histologically proven breast carcinoma who were treated with both NACT and surgery in Basavatarakam Indo American Cancer Hospital and Research Institute (BIACHRI) Hyderabad, Telangana, India were included in the study. Patients who underwent only surgery in BIACHRI with NACT done elsewhere, specimens with positive sentinel lymph node excision done before NACT and specimens with involved margin status after NACT were excluded from the study.
Fourteen cases (28%) were stage II tumours, 36 cases (72%) were stage III tumours (locally advanced breast cancers) at presentation. Twenty-seven (54%) out of these thirty-six stage III tumours were large operable breast cancers (cT3N1).
Before surgery these patients were treated with either anthracycline-based chemotherapy regimen with or without additional taxanes or targeted therapy with Trastuzumab (TZB). Anthracycline based therapy included adriamycin or doxorubicin and cyclophosphamide (AC) every three weeks for four cycles or received four cycles of Paclitaxel/Docetaxel (T) in addition after completion of AC regimen. Minimum of four cycles and maximum of eight cycles were given. Targeted therapy included four cycles of TZB with twelve cycles of Paclitaxel.
Data regarding standard preoperative investigations including initial biopsy was obtained from hospital laboratory information system. Baseline and post NACT mammogram findings, Estrogen Receptor (ER), Progesterone Receptor (PR), human epidermal growth factor receptor 2 (HER2/neu) status were noted where ever available. Hormonal receptor was considered as positive when more than 1% of neoplastic cells exhibited nuclear staining. The HER 2 Immunohistochemistry (IHC) was considered positive when the score was 3+ [12].
Grossing and microscopic assessment methods were adapted from the video tutorial available from MD Anderson website [8] and also from the standard protocol described by Provenzano E et al., and Bossuyt V et al., [7,11].
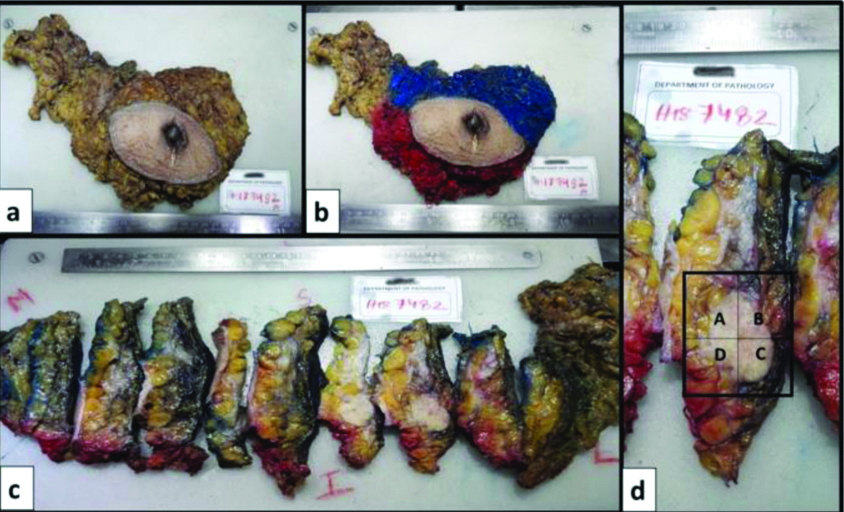
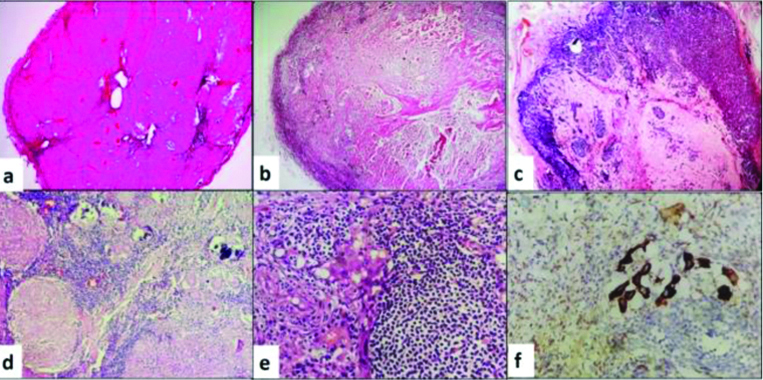
Grossing methodology: Gross findings were associated with radiological findings. Formalin fixed specimen was oriented followed by documentation of three-dimensional measurements of external surface [Table/Fig-1a]. The specimen was inked using different colours for anterosuperior, anteroinferior and posterior surfaces as shown in [Table/Fig-1b] to maintain the orientation. Specimen was serially sliced at 5 mm thickness to expose largest cross section of tumour bed [Table/Fig-1c]. All the slices were placed in order and were palpated carefully to feel for tissue densities or clips. Measurement of tumour bed (including tumour) and distance from margins were documented. Sections representing full face of tumour bed were studied for tumours less than 5 cm. For large tumours, 5 representative sections were taken initially. Extensive regrossing of tumour bed was done if initial sections were negative for residual tumour. A diagrammatic map was made for sections taken [Table/Fig-1d]. Sections were subjected to routine paraffin processing and Haematoxylin and Eosin (H&E) staining.
Grossing methodology. (a) Right Modified Radical Mastetomy (MRM) specimen; (b) Inking of superior, inferior and posterior surfaces with different colours; (c) Serial slicing at 5 mm thickness and identifying largest cross section of tumour bed; (d) Representative sections taken including the entire cross section of tumour bed with mapping of sections.
Microscopic evaluation: Analysis was performed individually by two senior pathologists, who has expertise in breast pathology and a junior pathologist. Consensus opinion was noted in case of discrepancies.
The specimens were assessed for residual tumour size, cellularity, grade, type, margins, lymphovascular invasion, perineural invasion, associated DCIS, number of axillary lymph nodes involved, measurement of largest deposit and therapy related changes. Final pathological diagnosis and treatment response categorisation was made according to the World Health Organisation (WHO) classification 5th edition 2019 and the tumour-node-metastases (ypTNM) staging system determined by using the 8th edition of the AJCC staging guidelines [5,13].
RCB score and class: The RCB score and class were determined by entering the following parameters in the MD Anderson Cancer Center’s online calculator [10].
The RCB scoring parameters included the following: (i) cellularity: it was the percentage of the tumour bed area that contains carcinoma (invasive and in situ). Cellularity was assessed by comparison with the charts provided in online calculator; (ii) estimate of the percentage of the carcinoma in situ in the tumour bed; (iii) two dimensions of the tumour bed containing residual cancer in millimeters; (iv) The number of residual-positive lymph nodes; (v) the longest diameter of the largest nodal metastatic deposit in millimeters [10]. The RCB classes with a class of 0 represents pCR and class 1-3 represent progressively greater extent of residual cancer.
Statistical Analysis
Descriptive analysis was used, data was expressed in percentages.
Results
This study included 50 female patients with age ranging from 32-67 (mean 50) years. A total of 20 (40%) patients were in the 41-50 years of the age group. Twenty-nine (58%) patients received four cycles of anthracycline drugs. Nineteen (38%) patients received four cycles of paclitaxel in addition. Two (4%) patients received TZB with paclitaxel in addition. Thirty-nine (78%) specimens were MRM and 11 (22%) were Breast Conservative Surgery specimens (BCS). PreNACT hormonal receptor and HER 2/neu status by IHC were available in 41 patients that included 19 (46%) triple negative tumours. [Table/Fig-2] summarises the clinicopathological parameters of all the patients included in the study.
Clinicopathological features of cases included in this study.
| Variables (n=50) | Values |
|---|
| Age (years) | 32-67 years (mean=50 years) |
| Pre NACT tumour size on digital mammogram (n=50) | 5-62 mm. mean=37 mm |
| Histological diagnosis | n (%) |
| Invasive carcinoma NST | 49 (98%) |
| Lobular carcinoma | 1 (2%) |
| Pre NACT tumour grade (n=50) | |
| 1 | 2 (4%) |
| 2 | 41 (82%) |
| 3 | 7 (14%) |
| Hormone receptor and HER 2 status in pre NACT core biopsy (n=41) |
| ER, PR, HER 2 negative | 19 (46%) |
| ER, PR positive, HER 2 negative | 12 (30%) |
| ER, PR negative, HER 2 positive | 5 (12%) |
| ER, PR, HER 2 positive | 5 (12%) |
| Chemotherapy regimens (n=50) | n (%) |
| 4 cycles AC | 29 (58%) |
| 4 cycles AC+4 cycles T | 19 (38%) |
| 12 cycles T+4 cycles TZB | 2 (4%) |
| Procedure | n (%) |
| Modified radical mastectomy | 39 (78%) |
| Breast conservation surgery | 11(22%) |
| Gross (n=50) | n (%) |
| Well defined residual tumour | 30 (60%) |
| Ill-defined fibrous bed with yellowish areas | 14 (28%) |
| Extensive fibrosis | 3 (6%) |
| Cystic change | 3 (6%) |
| Post NACT tumour size in mm (n=50) | 0-70 mm, mean=27 mm |
| Tumour staging (n=50) | |
| TIS | 2 (4%) |
| T0 | 11 (22%) |
| T1 | 15 (30%) |
| T2 | 15 (30%) |
| T3 | 5 (10%) |
| T4 | 2 (4%) |
NACT: Neoadjuvant chemotherapy; NST: No special type; ER: Estrogen receptor; PR: Progesterone receptor; HER 2: Human epidermal growth factor receptor 2, AC: Adriamycin, Cyclophosphamide regime, T-Paclitaxel; TZB: Trastuzumab; TIS: Carcinoma insitu
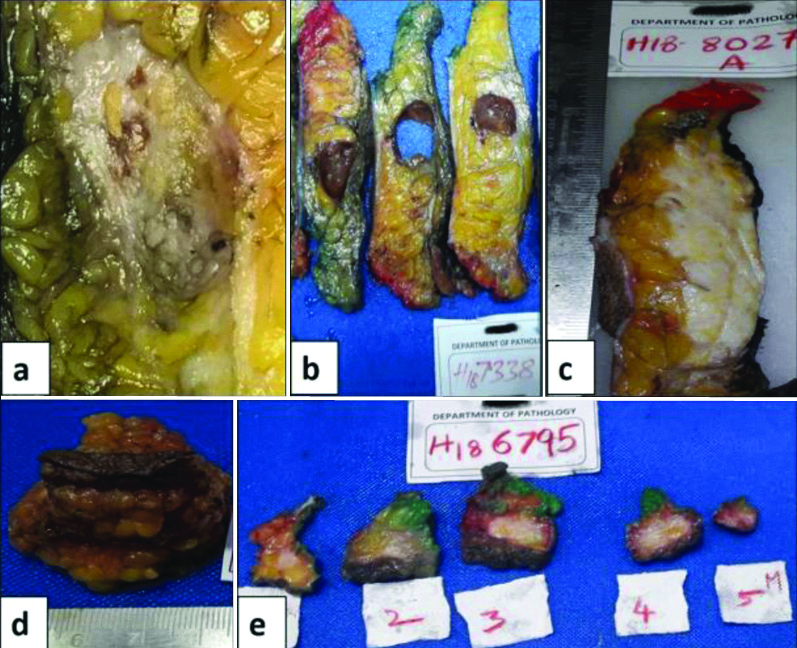
Well-defined tumour was identifiable in 60% specimens only. Rest of the specimens showed ill-defined fibrous areas and yellowish/haemorrhagic/calcific foci/cystic change. The various gross morphology features of tumour bed are given in [Table/Fig-3].
Variable gross morphologies of tumour bed post NACT.
(a) Ill-defined fibrosis with yellowish and haemorrhagic areas; (b) Cystic change; (c) Extensive fibrosis with no residual tumour; (d) and (e) BCS specimen, with fibrous appearance of tumour bed. Residual tumour was seen on microscopy
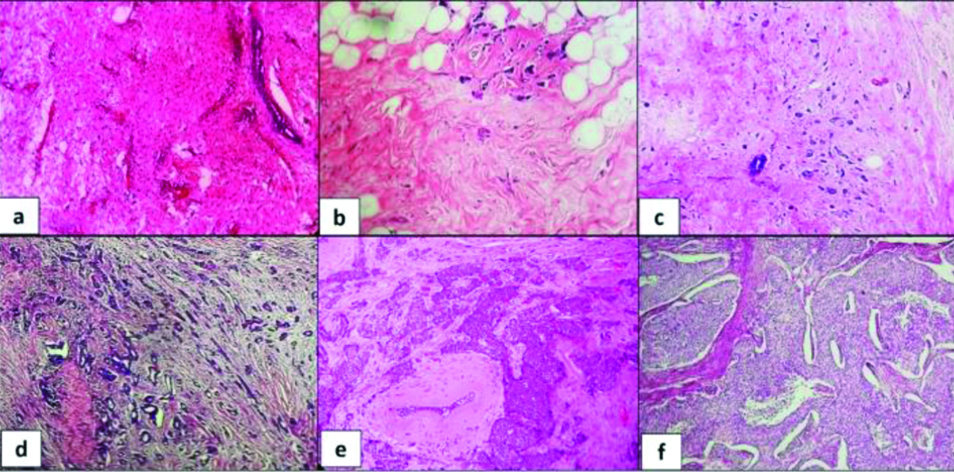
Microscopic analysis: Forty-nine (98%) specimens had invasive carcinoma (NST) and one (2%) was lobular carcinoma. Residual tumour cellularity varied from 0% to 95%. The various percentages of tumour cellularity are shown in [Table/Fig-4]. Breast parenchyma did not show residual tumour in twelve (24%) specimens which included nine (18%) with pCR (no invasive carcinoma in breast as well as lymphnodes) and three specimens with residual disease seen only in the lymph nodes. Five of the nine patients with pCR were triple negative. Three (6%) patients showed stable size or increase in tumour size during NACT and were considered as pathological No Response (pNR) category. Pathological response and RCB class are summarised in [Table/Fig-5].
Tumour bed cellularity post NACT; (a) No residual tumour. Tumour bed characterised by stromal fibroelastosis with prominent vessels, chronic inflammatory cells and paucity of ducts, (H&E x4); (b) Residual tumour cellularity of 5% (H&E x4); (c) Residual tumour cellularity of 10% (H&E x4); (d) Residual tumour cellularity of 40% (H&E x4); (e) Residual tumour cellularity of 60% (H&E x4); (f) Residual tumour cellularity of 90% (H&E x4).
Summary pathological response and RCB class in our study.
| RCB | pCR(%) | pPR(%) | pNR(%) |
|---|
| Class 0 | 9 (18%) | 0 | 0 |
| Class 1 | 0 | 5 (10%) | 0 |
| Class 2 | 0 | 17 (34%) | 1 (2%) |
| Class 3 | 0 | 16 (32%) | 2 (4%) |
RCB: Residual cancer burden; pCR: Pathological complete response; pPR: Pathological partial response; pNR: Pathological no response
[Table/Fig-6] summarises the clinicopathological features of cases with pCR. An increase in tumour grade was seen in 15 (39%) of the 38 specimens with residual carcinomas. Tumour grades before and after NACT were summarised in [Table/Fig-2,7].
Clinicopathological features of cases with pCR.
| Case | Age (years) | Laterality | Site | Pre NACT biopsy grade | NACT regimen | Procedure | DCIS (post NACT) | ER% | PR% | HER 2 score |
|---|
| 1 | 57 | Right | UOQ | 3 | 12T+4TZB | MRM | Present | 0 | 0 | 3 |
| 2 | 38 | Right | UOQ | 2 | 4AC+4T | BCS | Not seen | 0 | 0 | 1 |
| 3 | 49 | Right | LIQ | 2 | 4AC | BCS | Not seen | 60 | 4 | 0 |
| 4 | 51 | Right | UOQ | 2 | 4AC+4T | MRM | Not seen | 70 | 0 | 1 |
| 5 | 66 | Left | UOQ | 2 | 6AC | MRM | Not seen | 0 | 0 | 0 |
| 6 | 37 | Right | UOQ | 3 | 4AC | BCS | Not seen | 40 | 30 | 0 |
| 7 | 52 | Right | RA | 2 | 4AC | MRM | Not seen | 0 | 0 | 0 |
| 8 | 59 | Left | RA | 2 | 4AC+4T | MRM | Not seen | 0 | 0 | 1 |
| 9 | 41 | Left | RA | 3 | 4AC | MRM | Not seen | 0 | 0 | 0 |
UOQ: Upper outer quadrant; LIQ: Lower inner quadrant; RA: Retroareolar; T-Paclitaxel; TZB: Trastuzumab; AC: Adriamycin cyclphosphamide; MRM: Modified radical mastectomy; BCS: Breast conservation surgery; DCIS: Ductal carcinoma in situ
Summary of tumour grades before and after NACT.
| Post NACT | Pre NACT grade 1 (n=2) | Pre NACT grade 2 (n=41) | Pre NACT grade 3 (n=7) |
|---|
| NRT (n=12) | 0 | 8 | 4 |
| Grade 1 (n=1) | 1 | - | - |
| Grade 2 (n=20) | 1 | 19 | - |
| Grade 3 (n=17) | - | 14 | 3 |
NACT: Neoadjuvant chemotherapy; NRT: No residual tumour in breast
Most common treatment related histopathological changes seen were fibroelastosis (96%), followed by necrosis (38%), foamy histiocyte collections and degenerative changes in the tumour cells. More than one change was seen in each specimen. The nuclear changes included marked nuclear enlargement with hyperchromasia, pleomorphism and karyorrhexis. Cytoplasmic changes included foamy, mucinous and oncocytic change. Lymph nodes showed fibrosis and necrosis, granulomas and calcifications. In two (4%) of specimens, ITC were seen in lymph node mimicking histiocytes. A pan keratin immunostaining was performed to highlight tumour cells. [Table/Fig-8] summarises the therapy related changes. Therapy related changes seen in the tumour cells, stroma and lymph nodes are shown in [Table/Fig-9,10 and 11], respectively.
Summary of therapy related changes.
| Therapy related histopathological changes | Number of cases (%) |
|---|
| Fibroelastosis | 48 (96%) |
| Necrosis | 19 (38%) |
| Degenerative nuclear changes | 8 (16%) |
| Cytoplasmic changes (foamy, mucinous, oncocytic) | 11 (22%) |
| Foamy histiocytes | 19 (38%) |
| Chronic inflammatory cells | 7 (14%) |
| Haemosiderin laden macrophages | 5 (10%) |
| Calcifications | 6 (12%) |
| Foreign body giant cell reaction | 3 (6%) |
| Myxoid change in the stroma | 3 (6%) |
| Cystic change | 3 (6%) |
| Lymphnode changes | 12 (24%) |
| Fibrosis | 5 (10%) |
| Necrosis | 4 (8%) |
| Granulomas | 2 (4%) |
| Calcifications | 2 (4%) |
Therapy related changes of tumour cells: (a) Tumour cells with degenerative nuclear hyperchromasia (H&E, x40); (b) Tumour cells with degenerative nuclear atypia, karyorrhexis and necrosis (H&E, x40); (c) Tumour cells with foamy, vacuolated cytoplasm and intra cytoplasmic mucin. Stroma shows dense lymphocytic infiltrate (H&E, x40); (d) Tumour cells with oncocytic change (H&E, x 40); (e) Tumour bed with DCIS. No residual invasive carcinoma was seen in this case (H&E, x10); (f) Invasive lobular carcinoma (H&E, x10).
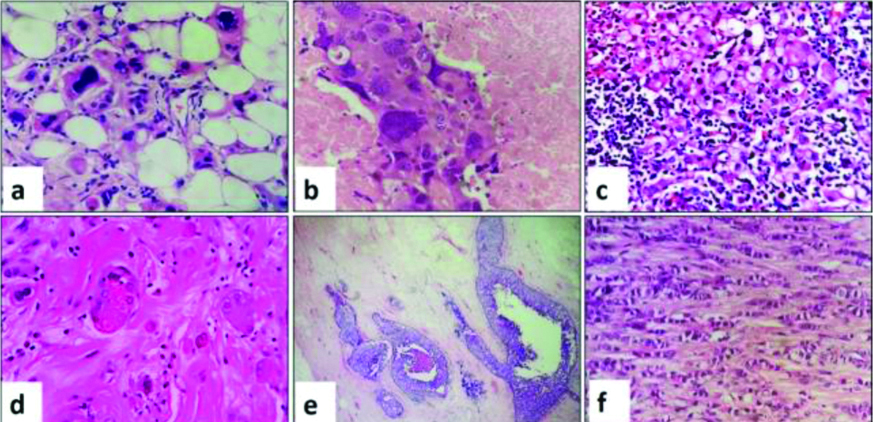
Therapy related stromal changes (a) Sheets of foamy macrophages admixed with lymphocytes (H&E, x40); (b) Haemosiderin laden macrophages (H&E x40); (c) Myxoid change in stroma (H&E x40); (d) Foreign body giant cell reaction (H&E x40).
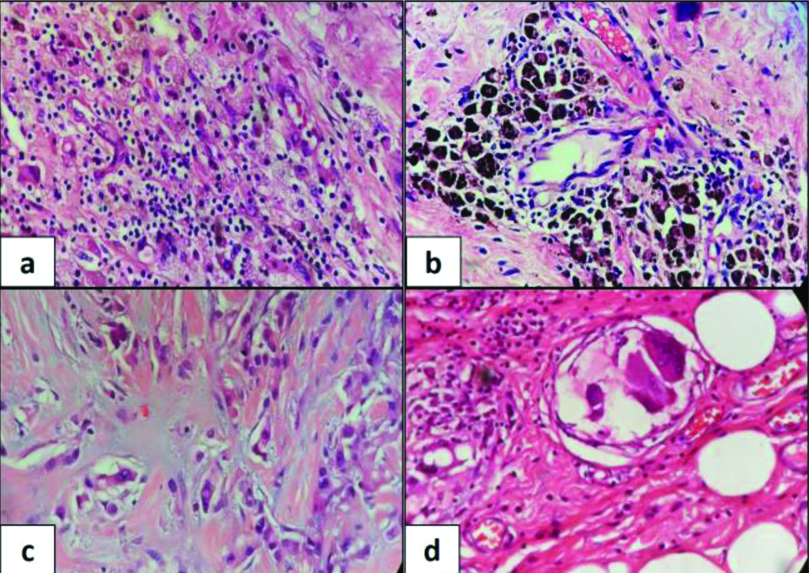
Therapy related changes in lymph node. (a) Fibrosis (H&E, x4); (b) Necrosis (H&E, x4); (c) Residual tumour with large areas of fibrosis (H&E, x4); (d) Granuloma with calcification (H&E, x4); (e) Metastatic ITC with histiocyte like morphology (H&E, x10); (f) ITC highlighted by pan keratin immunostain (IHC x40).
Follow-up data was available for forty-seven out of fifty cases for duration of 3 months to 22 months on an average of 10.5 months. Two (4%) patients developed chest wall recurrence only, five (11%) patients developed distant metastasis and one patient (2%) developed both. None of the cases with pCR had recurrence or metastasis.
Discussion
The pathologic response to NACT in 50 patients with breast carcinoma was assessed using RCB system developed by MD Anderson institute. The NACT aims to down stage the tumour and increase the rate of BCS. The rate of BCS in this study was 22% which was comparable to the earlier reports by van der Hage JA et al., (21.6%) and Scholl SM et al., (34%) [14,15]. However, Sharma K et al., reported BCS in 62% in their study [16]. Invasive carcinoma, NST was the predominant histological type (98%) and only one case of lobular carcinoma was seen. These observations were similar with those in the previous studies [17-19].
Gross examination revealed a well-defined tumour in only 60% of specimens in the present study whereas in the other specimens, there was an ill-defined tumour bed. Correlation with a pre-operative mammogram helped in locating the tumour bed in such cases. However, the ideal method in such circumstances would be a specimen radiograph which highlights even a tiny focus of residual tumour or a previously inserted clip. The latter also is ideal for mapping the sections. Other methods like photograph and manual methods can be used for this purpose [7].
The pCR is defined as absence of residual invasive carcinoma in both breast and axillary lymph nodes [5,7]. Presence of DCIS in the absence of invasive carcinoma is compatible with pCR. Presence of axillary lymph node deposits including ITCs excludes pCR [5,7]. In the present study, pCR (RCB class 0) was achieved in 9 patients (18 %) after NACT; these observations were comparable to other studies with pCR rates as 8 to 40.4% [18-20]. Various studies from India by Sheereen S et al., Sethi D et al., Pasam RK et al., reported pCR rates as 17.9%, 10% and 20%, respectively [17,20,21].
The pPR is further stratified into RCB classes I, II and III which indicate minimal, moderate and extensive residual disease respectively. Patients with minimal residual disease (RCB-I) carry prognosis similar to those with pCR (RCB-0) and patients with extensive residual disease (RCB-III) regardless of other parameters are associated with poor prognosis [8,22].
The therapy related changes seen commonly were fibrosis, necrosis, collections of foamy histiocytes, foamy/mucinous/oncocytic change in cytoplasm and degenerative nuclear changes. Tumour regressive changes were also seen in lymph nodes. Similar changes were documented in other studies [4,23]. Micro metastasis and ITCs in lymph nodes may be difficult to identify on morphology alone due to altered tumour cell morphology in lymph node. A pan keratin immunostain must be performed on lymph node in such cases as in two samples in the present study.
A careful and systematic sampling of post NACT specimens is needed as gross and microscopic morphological changes in these specimens are complex. Residual tumours are usually less well defined, soft and show heterogenous cellularity. So, sampling the full face of largest cross section of tumour bed is recommended rather random sampling in order to prevent false estimate of residual tumour. However, for large tumours five representative blocks may be sufficient [7]. The effect of NACT on tumour cellularity is not taken into consideration in American Joint Committee on Cancer (AJCC (y)) staging which is also difficult to apply on post NACT tumour beds due to presence of dispersed microscopic foci of carcinoma. Symmans WF et al., from MD Anderson cancer center proposed measurement of RCB as a continuous variable derived from the primary tumour dimensions, cellularity of the tumour bed, and axillary nodal burden which is available as a free online calculator and the parameters can be obtained from routine grossing and microscopic examination of specimen [10]. A standardised protocol for grossing as well as template for reporting post NACT breast specimens was published by Provenzano E et al., and Bossuyt V et al., after reviewing standard operating procedures used by 28 major breast cancer trials [7,11]. They also suggested RCB as preferred method for quantification of residual cancer among several other histopathologic classifications apart from AJCC(y) staging which has been described to categorise the tumour response to NACT [24-28]. The RCB has been shown to provide prognostic value independent of AJCC(y) stage for patients with post-treatment residual breast carcinoma [29].
Limitation(s)
The limitations of this study were lack of specimen radiography which is ideal method to locate residual tumour and small sample size.
Conclusion(s)
As pathological evaluation of tumour response post NACT is the gold standard compared to clinical and radiological response assessment, familiarity with gross and microscopic therapy related changes to accurately quantify the residual disease is important. The problem of sampling errors caused by heterogeneously variable cellularity was not addressed in AJCC(y) staging which can be overcomed by incorporating RCB score and class in the reporting. The parameters required for its calculation include two-dimensional tumour bed measurement, tumour bed cellularity, and percentage of DCIS, number of involved lymph node and size of largest metastasis. These could be applied in the present study without adding extra cost to the patient and the rate of pCR (RCB class 0) obtained was 18%. The detailed quantification of residual disease by RCB has prognostic significance independent of AJCC(y) staging; hence the present study highlights its feasibility in routine practice.
NACT: Neoadjuvant chemotherapy; NST: No special type; ER: Estrogen receptor; PR: Progesterone receptor; HER 2: Human epidermal growth factor receptor 2, AC: Adriamycin, Cyclophosphamide regime, T-Paclitaxel; TZB: Trastuzumab; TIS: Carcinoma insitu
RCB: Residual cancer burden; pCR: Pathological complete response; pPR: Pathological partial response; pNR: Pathological no response
UOQ: Upper outer quadrant; LIQ: Lower inner quadrant; RA: Retroareolar; T-Paclitaxel; TZB: Trastuzumab; AC: Adriamycin cyclphosphamide; MRM: Modified radical mastectomy; BCS: Breast conservation surgery; DCIS: Ductal carcinoma in situ
NACT: Neoadjuvant chemotherapy; NRT: No residual tumour in breast