The DIS are dedicated to provide information about drugs and pharmacotherapy, on request from other health care professionals, organisations or patients. It provides accurate, unbiased, factual information, in response to patient specific drug problems as received from various specialities of the health care system [1].
DIS is one of the twelve strategies recommended by World Health Organisation (WHO) to promote rational use of medicines [2]. It caters the services to all health care professionals and can be accessed by telephone, intranet and direct access [1]. Clinical pharmacologists play an important role in enforcement of rational use of medicines by providing the clinicians with authentic and updated information [1,3-5], based on evidence based medicine [2] from sources like drug label information, standard treatment guidelines and case reports.
Increasing incidence of infections caused by multidrug resistant organisms in patients with multiple co-morbidities call for individualised antimicrobial therapy [6]. An understanding of pharmacokinetic and pharmacodynamic principles of antimicrobials is required to provide information regarding appropriate antimicrobial therapy. The expertise of clinical pharmacologists aids in selection of appropriate antimicrobial based on both culture sensitivity reports and knowledge of drug penetration at site of infection [7].
Other important services of DIS include suggestion of alternative drug therapy in case of intolerance or resistance to first-line drugs, recommendation of dosage adjustments in cases with hepatic and/or renal impairment and identification of drug-drug interactions [3,5]. In combination chemotherapy, it is difficult to identify the offending drug in case an adverse drug reaction occurs. DIS can aid in identifying the offending drug by dechallenge and rechallenge procedures [4,8].
As the roles of DIS are diverse, like suggesting appropriate antimicrobial therapy, identification of adverse drug reactions, drug-drug interactions, recommendation of dosage modifications and also WHO recommended DIS as one of the twelve strategies to promote rational use of medicines. This study was done to understand the impact of DIS on promotion of rational use of antimicrobial agents, at a tertiary care hospital.
Materials and Methods
This descriptive retrospective study was done at Department of Clinical Pharmacology and Therapeutics (CP&T) at a tertiary care superspecialty hospital based on case referrals during January 2014-June 2018. The Department provides DIS to the clinicians of the institute since 2013. As this is a qualitative study (descriptive in nature) on the basis of feasibility [9], 269 case referrals done during the study period were analysed in Sep 2018 to include 126 infection related queries in the study.
Inclusion criteria: Clinicians refer cases to DIS for drug related therapeutic issues. Cases with infection related medication issues requiring opinion related to appropriate antimicrobial therapy based on culture sensitivity reports, alternative drug therapy in cases of intolerance or resistance to first line drugs, dosage adjustments in cases with hepatic and/or renal impairment and suspected drug to drug interactions, were included in the study.
Exclusion criteria: Cases other than infection related queries were excluded from the study.
Patient characteristics like demographic details, presenting complaints, clinical history, co-morbid conditions and clinical diagnosis captured in the case details form were analysed. In addition, detailed information about cultures sent and culture/susceptibility results, the treatment offered by the prescribing physician, reason for referral and suggestions given by the DIS captured in the case details form were also analysed.
Referrals related to drug-to-drug interactions and suspected adverse drug reactions were analysed using WHO-Uppsala Monitoring Centre (UMC) scale of causality assessment [8] and thereafter managed by suggesting an appropriate alternative strategy to establish a safer and effective regimen. Adverse drug reactions thus identified were recorded and reported to the Pharmacovigilance Programme of India (PvPI).
Statistical Analysis
The data was presented as mean±SD for quantitative variables and proportions for categorical variables. The number of antimicrobials prescribed per case before and after DIS opinion were compared using paired t-test considering p<0.05 as significant.
Results
DIS received a total number of 269 referrals during the 4.5 years period of study, of these, 126 (46.8%) cases with mean age of 35±18.2 years, related to infections and antimicrobial usage were analysed in the study. Demographic and clinical characteristics of cases are presented in [Table/Fig-1].
Showing the characteristics of the cases included in the study.
| Characteristics | n=126 |
|---|
| Age in (years) (Mean±SD) | 35±18.2 |
| Gender |
| Male n, (%) | 60 (47.6) |
| Female n, (%) | 66 (52.4) |
| Departments |
| Medical departments n, (%) | 86 (68.3) |
| Surgical departments n, (%) | 40 (31.7) |
| Laboratory investigations |
| Cultures sent n, (%) | 126 (100) |
| Blood culture n, (%) | 31 (24.6) |
| Urine culture n, (%) | 13 (10.3%) |
| Other specimens n, (%) | 82 (65.1) |
| Reports |
| Culture positive n, (%) | 82 (65.1) |
| Gram positive n, (%) | 15 (18.3) |
| Gram negative n, (%) | 45 (54.9) |
| Fungal isolates n, (%) | 17 (20.7) |
| Others n, (%) (included Colonisers) | 5 (6.1) |
| Culture negative n, (%) | 28 (22.2) |
| Culture repeat advised n, (%) | 16 (12.7) |
Of the 126 cases, 82 were culture positive. The data of these culture positive cases was analysed. Among the culture positives, 43 samples reported MDR organisms that included Methicillin Resistant Staphyllococcus aureus (MRSA) (6), extended spectrum beta-lactamase (ESBL) Escherichia coli (8), ESBL Klebsiella pneumoniae (12), Pan Drug Resistant (PDR) K Pneumoniae (3), Acinetobacter baumanni (6), Burkloderia cepacia (7) and Stenotrophomonas maltophila (1). Among isolated organisms, susceptibility to beta-lactams was seen in 19 (23.1%), colistin 16 (19.4%), cotrimoxazole 9 (10.9%), macrolides 8 (9.7%), aminoglycosides 9 (10.9%) and clindamycin 2 (2.4%).
Of the 126 cases, 53 (42.1%) were referred for opinion regarding appropriate antimicrobial therapy based on culture/susceptibility report followed by 27 (21.5%) was referred for opinion regarding dosage adjustment of antimicrobials [Table/Fig-2].
Showing the details of case referrals to Drug Information Services (DIS).
| S. No. | Reason for referral (N=126) | n (%) |
|---|
| 1 | Appropriate antimicrobial therapy based on culture/susceptibility report | 53 (42.1%) |
| a. Mean number of antimicrobials prescribed before DIS opinion | 3.4±1.85 |
| b. Mean number of antimicrobials prescribed based on DIS opinion | 1.62±1.38 |
| 2 | Suspected adverse drug reaction and rechallenge opinion | 18 (14.3%) |
| a. Drug induced hepatotoxicity | 6 |
| b. Ethambutol induced acute pancreatitis | 1 |
| c. Rifampcin induced thrombocytopenia | 2 |
| d. ATT induced leucopenia | 1 |
| e. Drug induced rash | 4 |
| f. Drug induced acute kidney injury | 2 |
| g. Clindamycin induced diarrhea | 1 |
| h. Levofloxacin induced delirium | 1 |
| 3 | Dosage adjustment of antimicrobials | 27 (21.5%) |
| For hepatic impairment | 4 |
| a. Caspofungin | 1 |
| b. Voriconazole | 1 |
| c. ATT | 2 |
| For renal impairment | 23 |
| a. Colistin | 7 |
| b. Fluconazole | 3 |
| c. Meropenem | 4 |
| d. Vancomycin | 3 |
| e. Cotrimoxazole | 3 |
| f. Ethambutol and Pyrazinamide | 3 |
| 4 | Suspected drug-drug interactions | 12 (9.5%) |
| Pharmacodynamic interactions | 6 |
| Pharmacokinetic interactions | 6 |
| 5 | Follow-up calls | 16 (12.7%) |
ATT: Anti-tubercular therapy
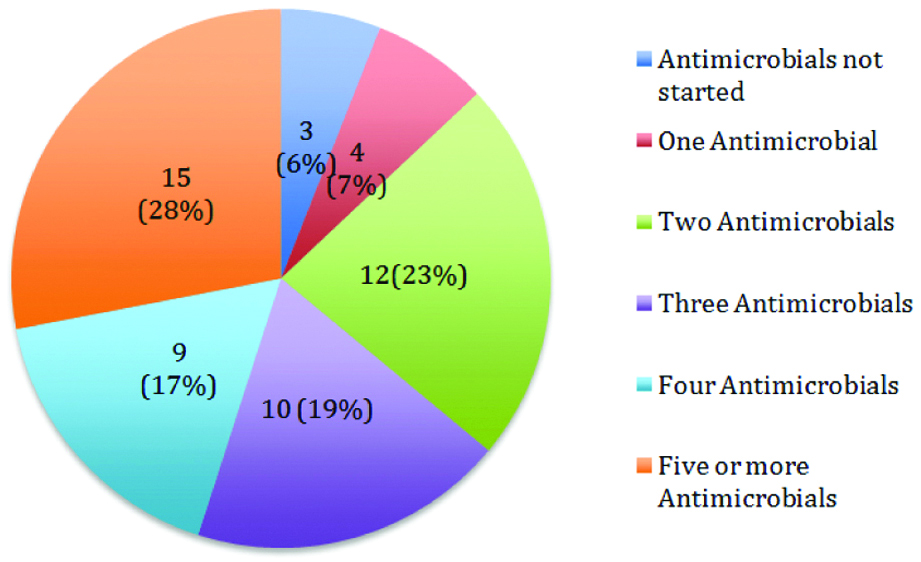
Number of antimicrobials prescribed as empiric antimicrobial therapy in cases referred for opinion on antimicrobial therapy was estimated and analysed. As depicted in [Table/Fig-3] it was observed that of 53 cases, ≥ three antimicrobials were prescribed in 34 (64%) and less than three antimicrobials were prescribed in 16 (30%) cases.
Pie chart showing of the number of antimicrobials prescribed as empiric antimicrobial therapy before DIS opinion (n=53).
Appropriate drug therapy was advised based on culture susceptibility reports and standard treatment guidelines. With recommendation of DIS, the number of antimicrobials got reduced to single agent in 10 cases (19%) and to two antimicrobials in 21 cases (40%). In five cases (9%), antimicrobials were stopped and in the rest 17 cases (32%) more than two antimicrobials were suggested [Table/Fig-4].
Pie chart showing of the number of antimicrobials prescribed as definitive antimicrobial therapy after DIS opinion (n=53).
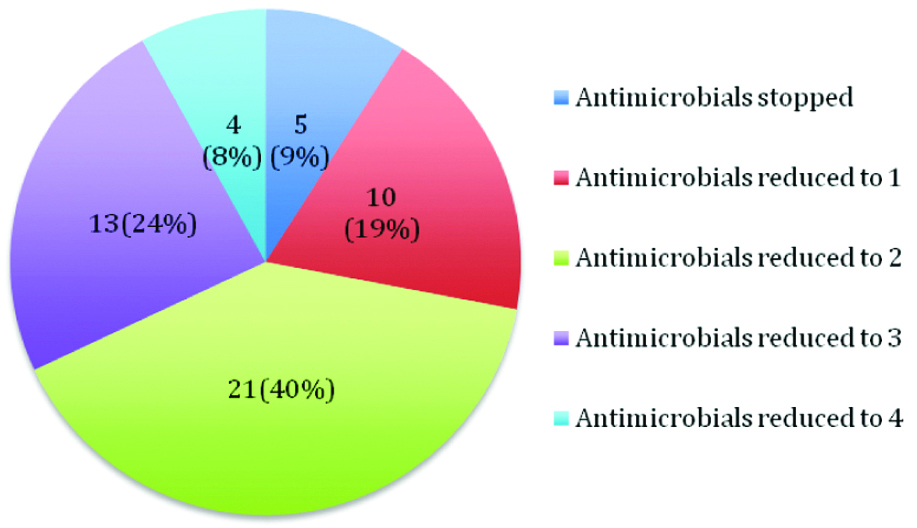
In these 53 cases, mean number of antimicrobials prescribed per patient as empiric antimicrobial therapy before case referral to DIS was 3.4±1.85 (including ATT and ART). And the mean number of antimicrobials prescribed reduced to 1.62±1.38 per patient (p<0.001 using paired t-test) based on DIS recommendations [Table/Fig-3,4].
The different classes of antimicrobials prescribed were beta-lactams (36), colistin (22), clindamycin (8), cotrimoxazole (7), macrolides (4) and aminoglycosides (15). Other antimicrobials prescribed included fluoroquinolones, glycopeptide inhibitors (vancomycin and teicoplanin), linezolid, tetracycline, anti-tubercular drugs and anti-retroviral drugs. Sixteen culture-negative cases were referred for opinion on appropriate antimicrobial therapy, empiric treatment in these cases was advised based on standard treatment guidelines.
In 18 cases, drug induced adverse drug reactions were suspected and were reported to PvPI. The adverse drug reactions observed are listed in [Table/Fig-2]. Among the 18 cases, 6 developed drug induced hepatotoxicity, of which it was ATT induced hepatotoxicity (5) while in one case it was statin induced. Drug induced rash was observed in four cases, in one case it was Isonicotinic Acid Hydrazide (INH) induced, in other case it was vancomycin induced and in rest two cases it was beta-lactam induced. Another adverse drug reaction was acute kidney injury induced by amphotericin B (1) and colistin (1). Among these adverse drug reactions reported, causality was assessed as certain in four cases and as probable in rest of the 14 cases using WHO-UMC scale of causality assessment [8].
Dosage adjustments were recommended for drugs enumerated in [Table/Fig-2] in four patients with hepatic impairment and 23 cases with renal impairment [10,11]. However, in one case, levofloxacin was stopped in a case with pre-existing hepatic dysfunction in view of rising bilirubin levels. Other antimicrobials for which dosage adjustment was suggested included imipenem+cilastatin, cefaperazone+sulbactam, lamivudine, doxycycline and amikacin as there were evidence of renal dysfunction.
Drug-Drug Interactions were suspected in 12 cases. Among them six were pharmacodynamic interactions and rest were pharmacokinetic interactions. Most of the pharmacodynamic interactions were due to prescription of two antimicrobials with either same mechanism of action or same antimicrobial spectrum like linezolid+vancomycin, vancomycin+teicoplanin, vancomycin+cloxacillin and meropenem+dicloxacillin. In one case, it was advised to stop amikacin in a patient on amphotericin B to prevent synergistic nephrotoxicity. The suspected pharmacokinetic interactions included rifabutin+ritonavir, rifampcin+nevirapine, rifampcin+clarithromycin, rifampicin+acitrom, fluconazole+acitrom and ATT+phenytoin [12]. In all these cases, appropriate plan of management was suggested on case-by-case basis.
Among 12 cases with Drug-Drug Interactions (DDI), adverse reactions were observed in two cases. In a case, renal impairment developed due to amphotericin B+Amikacin, hepatic dysfunction developed in a case on rifabutin+ritonavir. In all the other cases, due to early intervention, adverse events were prevented [13].
For all these cases, follow-up was done and it was observed that clinicians modified the drug regimens as recommended by DIS and had good clinical outcomes. Inadvertent effects were not observed in any of the cases.
Discussion
This observational study was undertaken to evaluate the impact of DIS on the promotion of rational use of antimicrobials in tertiary care hospital. With DIS recommendations, the number of the antimicrobial agents prescribed per patient significantly decreased. DIS thus contributed to promotion of rational use of antimicrobials by reducing the usage to ≤2 antimicrobial agents by providing unbiased, factual drug information based on culture susceptibility reports and standard treatment guidelines.
For all the 126 infection related cases, DIS were provided to clinicians, as it is a hospital-based service. Nova-Manosalva MA et al., assessed activities of 129 drug information centers in 18 European countries [14]. and a study done at Ethiopia also assessed activities of DICs [15].
Reported list by 2019 Centers for Disease Control and Prevention (CDC) Antibiotic Resistant Threats classifies eighteen antibiotic-resistant bacteria and fungi into three categories based on level of concern to human health as urgent, serious, and concerning [16,17]. In this study, 43 cultures (34.1%) reported MRSA, ESBL E.Coli, ESBL K. pneumoniae, Pandrug Resistant (PDR) K. Pneumoniae, Acinetobacter baumanni, Burkloderia cepacia and Stenotrophomonas maltophila. Mechergui A et al., reported a significant increase in the incidence of the MDR bacteria in a study done on immunocompromised patients [6]. As Antimicrobial Resistance (AMR) is a global crisis, fighting this threat is a public health priority. Improving rational use of antimicrobials and laboratory capacity to identify resistant microorganisms are among the core strategies recommended to prevent resistant infections and their spread [16].
In this study, majority of cases were referred to DIS for opinion regarding appropriate antimicrobial therapy based on culture/susceptibility report, followed by queries related to dosage adjustment of antimicrobials and suspected ADRs. A study done by Bhawsar R et al., in India [18], a study done by Almazrou DA et al., [19] and a study done by Tefera YG et al., in Ethiopia [15] too assessed the type of queries received, the details of which are presented in [Table/Fig-5].
Comparison between different studies reporting activities of DIS.
| S. No. | Study | Relevant details | Drug Information Services (DIS) |
|---|
| Query Resolution Service- End-User |
| 1 | Nova-Manosalva MA et al., 2016 [14] | Analysed 129 DICs in 18 European countries | 56% of DICs- cater services toHealth care professionals43% of DICs- cater services toHealth care professionals and General Public |
| 2 | Tefera YG et al., 2019 [15] Ethiopia | Ethopian study | Query resolution service45.3% to pharmacists11.3% to General Public |
| 3 | This study (n=126) | Hospital based DIS | Query resolution serviceOnly to physicians |
| Reason for referral to DIS |
| 1. | Bhavsar R et al., 2012 [18] | Majority of queries | Appropriate drug therapy/Choice of therapy |
| 2. | Almazrou DA et al., 2017 [19] | 50% of queries | Dosage and administration of drugs |
| 3 | Tefera YG et al., 2019 [15] | 23.5% of queries8.3% of queries7.9% of queries | AntibioticsAnalgesicsSteroid |
| 4 | This study(n=126) | 42.1% of queries21.5% of queries14.3% of queries9.5% of queries12.6% of queries | Appropriate antibiotic therapyDosage adjustmentSuspected ADRs and Rechallenge opinionSuspected drug-drug interactionsFollow-up related |
DIC: Drug information centre; ADRs: Adverse drug reactions
Due to the increasing incidence of infections caused by multidrug resistant organisms, the empiric treatment by clinicians often includes broad-spectrum antimicrobials. This is to cover all suspected gram-positive and gram-negative organisms along with coverage for anaerobic organisms and fungal infections based on site of infection and presence of immuno-compromised states and comorbidities. Owing to all these, in this study, among 53 cases, empiric treatment comprised of more than five antimicrobials in 15 (28%) cases and 4 antimicrobials were prescribed in 9 (17%) cases and 3 antimicrobials were prescribed in 10 (19%) (which included ATT in 21 cases and ART in 8 cases). In just 3 (6%) cases, antimicrobials were not started and one antimicrobial prescribed in 4 (7%) cases. Similar concerns were expressed in ICMR, Scoping report on AMR in India, in which it was opined that broad-spectrum antibiotics are being prescribed empirically to cover all possible illnesses, to avoid increase in the severity of illness. It reported an increase in use of third generation cephalosporin and carbapenems and a decrease in the use of narrow-spectrum penicillin. It also reported an increase in use of carbapenems owing to increased incidence of ESBL producing organisms [20].
To the best of knowledge, this is the first study that was done to assess whether DIS can promote rational use of drugs, as DIS is recommended by WHO as one of the strategies to promote rational use of drugs. This study demonstrated that DIS contributed to a significant reduction in mean number of antimicrobials prescribed (p<0.0001) by suggesting appropriate antimicrobial therapy. Suspected ADRs were reported to PvPI, and on case-by-case appropriate management of ADRs with sequential rechallenge was suggested where required. Early identification of suspected drug-drug interactions and recommendation of dosage adjustments [13] has prevented development of adverse events including events due to therapeutic failure. DIS thus helped to promote rational use of antimicrobials.
Polypharmacy owing to multiple co-morbidities in an individual patient and isolation of resistant microorganisms prompted calls to DIS and the recommendations of DIS ensured rational use of antimicrobials beyond doubt. WHO also endorsed that provision of independent, unbiased information by DICs and drug bulletins is essential to promote rational use of drugs using evidence based medicine [2]. Clinical pharmacologists provided DIS by personally attending all the referrals, after obtaining complete medication history, clinical status, culture reports, indications for different medications and treatment prescribed. This ensured the completeness of the data collected. Irrespective of the query raised, each prescription was holistically reviewed by the DIS and all relevant suggestions were personally communicated to the treating clinician and cases were followed-up meticulously.
Limitation(s)
This was a descriptive retrospective study, hence results were based on previously collected data.
Conclusion(s)
DIS is a very useful resource, which provides unbiased, factual drug information to clinicians and patients. They must be encouraged and established in all healthcare facilities, as they strengthen rational use of antimicrobials.
ATT: Anti-tubercular therapy
DIC: Drug information centre; ADRs: Adverse drug reactions