Enterococci are gram positive cocci that occur mainly in pairs but also in short chains and produce smooth, gray colonies that are either non-haemolytic or alpha haemolytic [1,2]. They were distinguished from streptococci and related taxonomy by their ability to grow at 10°C and 45°C, and growth in the presence of pyrrolidonylarylamidase [1]. Various pathogenic species of enterococci are E.avium, E.faecalis, E.raffinosus, E.malodoratus, E.pseudoavium, E.solitarius, E.gallinarum, E.faecium, E.casseliflavus, E.mundtii, E.durans, E.hirae [3]. Risk factors for infections include frequent exposure to antimicrobial agents particularly the use of vancomycin and third generation cephalosporins, decreased immunity or neutropenia, renal insufficiency, use of steroids and presence of an indwelling urinary catheter [4,5]. Vancomycin resistant enterococci infection rates are highest among critically ill patients admitted in Intensive Care Units (ICU) with limited treatment options [6]. Infections by enterococci have historically been treated with semi-permeable membrane active agents (e.g., penicillin or ampicillin) in association with an aminoglycoside (streptomycin/gentamicin); however such combination treatment has failed to work due to emergence of resistance such as HLAR, beta-lactam antibiotics resistance or vancomycin resistance [7]. There are very few data regarding enterococcal infections in Manipur [8]. Hence, this study was taken up with the objective of generating data on speciation of enterococci and their antibiogram pattern.
Materials and Methods
The present study was a cross-sectional study conducted in the Department of Microbiology of a Tertiary Care Hospital of North East India, Jawaharlal Nehru Institute of Medical Sciences (JNIMS), Manipur, Imphal, India from September 2018 and August 2020. Informed written consent (prescribed format) was obtained from participating individuals and in case of minors, assent was taken from the parents/legal guardians. Approval of ethical committee was obtained from the Institutional Ethical Committee JNIMS vide no. Ac/0/IEC/JNIMS/2018/(PGT).
Inclusion criteria: Patients of all age groups and genders with history of urinary tract infection, presence of prolonged urinary catheterisation and wound infection attending out-patient, and in-patient department of medicine, surgery, obstetrics and gynaecology, paediatrics, orthopaedics and ICU, JNIMS were considered as study population.
Exclusion criteria: The specimens showing contaminants, presence of duplicate isolates, patients who refuse to take part in the study and faecal samples were excluded from the study.
Sample size was calculated by:
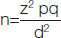
Where, n=sample size, z=1.96 at 95% confidence interval, p=prevalence from previous study=3.53% [9], q=100-p=96.47%, d=allowable error=3%. According to formula it came 145 (approx). So, the required sample size for this study was taken as 146 enterococcal isolates.
All the clinical samples except blood were processed initially by plating on Blood agar and MacConkey Agar, and incubating at 37°C for 18-24 hours. Blood samples were inoculated first in Brain Heart Infusion (BHI) broth, incubated at 35-37°C for five days, examined daily for microbial growth (turbidity) followed by subculture on blood agar and MacConkey agar. Identification of the enterococcal isolates were performed by adopting standard protocols such as cultural characteristics, gram stain, motility testing, catalase test, bile esculin test, salt tolerance test and α-pyrrolidonyl β-naphthylamide test (PYR). Further, speciation was carried out using the conventional scheme of Facklam and Collins [3].
The antimicrobial susceptibility testing was performed on Mueller Hinton agar plate by the Kirby Bauer disc diffusion method using the commercially available antimicrobial 6 mm discs (Himedia, Mumbai, India). The antibiotics tested were as follows- for urinary isolates and catheter tip ampicillin 10 μg, ciprofloxacin 5 μg, linezolid 30 μg, nitrofurantoin 300 μg, vancomycin 30 μg and teicoplanin 30 μg. For isolates from other sites like pus, wound, blood- ampicillin 10 μg, linezolid 30 μg, vancomycin 30 μg and teicoplanin 30 μg. High level gentamycin (120 μg) and high level streptomycin (300 μg) were used for all isolates [10].
Quality control: Every batch of media prepared was checked for sterility for 24 hours. Potency of disk used was checked with Enterococcus faecalis ATCC 29212.
Statistical Analysis
Data was analysed using descriptive statistics (percentage and proportion).
Results
During the study period of two years, 146 enterococci were recovered from 14114 different clinical samples, accounting for an infection rate of 1.03% of which 71 (49%) were from inpatient, 60 (41%) from out-patient and 15 (10%) from ICU. Among 146 enterococcal isolates, maximum number of isolates were obtained from urine 116 (79.5%) and minimum number from catheter tip 3 (2.1%) [Table/Fig-1]. The predominant isolates were E.faecalis (82.2%) followed by E.faecium (15.9%) and other species were E.durans (1.3%) and E.gallinarum (0.7%). Among the ICUs highest enterococcal infection was observed in Surgical Intensive Care Unit (SICU) 46.7% followed by Medicine Intensive Care Unit (MICU) 40% and Paediatric Intensive Care Unit (PICU) 13.3% [Table/Fig-2]. The distribution of Enterococcus spp. in various other departments is shown in [Table/Fig-3,4].
Total no. of Enterococcal isolates in various clinical samples.
| Enterococcus species (N=146) | Urine (%) | Blood (%) | Pus (%) | Wound swab (%) | Catheter tip (%) |
|---|
| E.faecalis (n=120) | 100 (83.3) | 6 (5) | 9 (7.5) | 3 (2.5) | 2 (1.7) |
| E.faecium (n=23) | 14 (60.9) | 6 (26) | 1 (4.3) | 1 (4.3) | 1 (4.3) |
| E.durans (n=2) | 1 (50) | 1 (50) | 0 | 0 | 0 |
| E. gallinarum (n=1) | 1 (100) | 0 | 0 | 0 | 0 |
| Total (n=146) | 116 (79.5) | 13 (8.9) | 10 (6.8) | 4 (2.7) | 3 (2.1) |
*n=No. of isolates
Distribution of clinical isolates in ICU (n=15).
| Enterococcus spp. (n=146) | MICU (%) | SICU (%) | PICU (%) |
|---|
| E.faecalis (n=120) | 4 (3.3) | 5 (4.2) | 1 (0.8%) |
| E.faecium (n=23) | 2 (8.7) | 2 (8.7) | 1 (4.3) |
| E.durans (n=2) | 0 | 0 | 0 |
| E.gallinarum (n=1) | 0 | 0 | 0 |
*n=No. of isolates, MICU: Medicine intensive care unit; SICU: Surgical intensive care unit; PICU: Paediatric intensive care unit
Distribution of clinical isolates in OPD (n=60).
| Enterococcus spp. (n=146) | Medicine (%) | Surgery (%) | Obstetrics and Gynaecology (%) | Paediatrics (%) |
|---|
| E.faecalis (n=120) | 40 (33.3) | 5 (4.2) | 5 (4.2) | 4 (3.33) |
| E.faecium (n=23) | 3 (13.0) | 0 | 3 (13.0) | 0 |
| E.durans (n=2) | 0 | 0 | 0 | 0 |
| E.gallinarum (n=1) | 0 | 0 | 0 | 0 |
n=No. of isolates
Distribution of clinical isolates in IPD (n=71).
| Enterococcus spp. | Medicine (%) | Surgery (%) | Obstetrics and Gynaecology (%) | Paediatrics (%) | Orthopaedics (%) |
|---|
| E.faecalis (n=120) | 31 (25.8) | 12 (10.0) | 1 (0.8) | 10 (8.3) | 2 (1.7) |
| E.faecium (n=23) | 5 (21.7) | 3 (13.0) | 1 (4.3) | 3 (13.0) | 0 |
| E.durans (n=2) | 1 (50) | 0 | 0 | 1 (50) | 0 |
| E.gallinarum (n=1) | 1 (100) | 0 | 0 | 0 | 0 |
n=No. of isolates
In this study, more number of cases was seen among female (69.2%) than male (30.8%) thus showing female predominance. The male:female ratio was found to be 1:2.2. Mean age of males was 41.8±22.28 and females were 33.59±18.29 [Table/Fig-5]. E.faecalis was found predominant in 21-30 years age group whereas E. faecium was highest in the age group of 31-40 years [Table/Fig-6].
Distribution of Enterococci among different age group and gender.
| Age groups (years) | No. of samples collected (%) | Gender |
|---|
| Males (%) | Females (%) |
|---|
| 1-10 | 16 (10.9) | 6 (13.3) | 10 (10.0) |
| 11-20 | 18 (12.3) | 3 (6.7) | 15 (14.8) |
| 21-30 | 30 (20.5) | 5 (11.1) | 25 (24.7) |
| 31-40 | 26 (17.8) | 6 (13.3) | 20 (19.8) |
| 41-50 | 18 (12.3) | 7 (15.6) | 11 (10.8) |
| 51-60 | 16 (10.9) | 8 (17.8) | 8 (7.9) |
| 61-70 | 17 (11.6) | 6 (13.3) | 11 (10.8) |
| 71-80 | 4 (2.7) | 3 (6.7) | 1 (0.9) |
| 81-90 | 1 (0.7) | 1 (2.2) | 0 (0) |
| Total | 146 | 45 (100) | 101 (100) |
| Mean age with standard deviation | 41.8±22.28 | 33.59±18.29 |
Distribution of Enterococcal isolates among different age groups.
| Enterococcal isolates | 1-10 yn=16 | 11-20 yn=18 | 21-30 yn=30 | 31-40 yn=26 | 41-50 yn=18 | 51-60 yn=16 | 61-70 yn=17 | 71-80 yn=4 | 81-90 yn=1 |
|---|
| E.faecalis (n=120) | 11 (9.1%) | 16 (13.3%) | 28 (23.3%) | 18 (21.6%) | 16 (13.3%) | 13 (10.8%) | 16 (13.3%) | 3 (2.5%) | 0 |
| E.faecium (n=23) | 4 (17.4%) | 1 (4.3%) | 2 (8.7%) | 8 (34.8%) | 2 (8.7%) | 2 (8.7%) | 1 (4.3%) | 1 (4.3%) | 1 (4.3%) |
| E.durans (n=2) | 1 (50%) | 1 (50%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E.gallinarum (n=1) | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 |
*n=No. of isolate
On studying the antibiotic susceptibility pattern it was found that most of Enterococcus isolates were predominantly resistant to ampicillin and ciprofloxacin (73.9% in both) followed by 10.3% to vancomycin, 7.5% to teicoplanin and 3.4% to linezolid. Out of the 120 E. faecalis isolates, 56 (46.6%) showed HLGR and HLSR in 76 (63.3%), whereas among 23 E. faecium HLSR was found in 18 (78.2%) isolates and HLGR in 12 (52.1%) as shown in [Table/Fig-7].
Antimicrobial susceptibility pattern of Enterococcus spp. done by Kirby Bauer disc diffusion method.
| Enterococcus spp. | AMP | CIP | VAN | TEI | NIT | LZ | HLG | HLS |
|---|
| S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R |
|---|
| E. faecalis | 30 | 90 | 27 | 75 | 107 | 10 | 105 | 10 | 60 | 34 | 117 | 3 | 64 | 56 | 44 | 76 |
| E. faecium | 7 | 16 | 3 | 12 | 17 | 4 | 21 | 1 | 10 | 6 | 21 | 2 | 11 | 12 | 5 | 18 |
| E. durans | 0 | 2 | 0 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 1 | 1 | 2 | 0 |
| E. gallinarum | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| Total (n=146) | 38 (26.1%) | 108 (73.9%) | 31 (21.2%) | 88 (73.9%) | 126 (84.9%) | 15 (10.3%) | 127 (87.6%) | 11 (7.5%) | 72 (60.5%) | 41 (34.4%) | 141 (96.6%) | 5 (3.4%) | 76 (52%) | 70 (47.9%) | 51 (34.9%) | 95 (65%) |
Note: As, the number of isolates are not equal, all the antibiotics are not used for all isolates; n=no. of isolates, AMP: Ampicillin; CIP: Ciprofloxacin; VAN: Vancomycin; TEI: Teicoplanin; NIT: Nitrofurantoin; LZ: Linezolid; HLG: High level gentamycin; HLS: High level streptomycin; *For urine (n=116) samples and catheter tip (n=3): AMP, CIP, LZ, NIT, VAN AND TEI were used; *For Blood (n=13), pus (n=10), wound swab (n=4): AMP, VAN, TEI, LZ were used; *HLG and HLS were used for all isolates ; *For Intermediate isolates (n=19): Vancomycin, Teicoplanin, Nitrofurantoin
Discussion
Enterococci are emerging as one of the most common agents of hospital acquired infection. At this context, there is a need to isolate, identify, speciate enterococci, study their antibiogram among the clinical isolates. In this study, enterococcal strains constituted 1.03% of infection. The prevalence of enterococcal infection in other Indian studies were 1.49% (Taneja N et al.,) from a hospital in western India, 1.16% (Mendiratta DK et al.,) from a rural hospital of Central India and 3.5% (Mathur S) from a tertiary care hospital of Northern India [9,11,12]. [Table/Fig-8] shows few studies published from North-east India showing the prevalence of 1.01% [8], 7.47% [13].
Prevalence of enterococcal infection in studies from North-east India.
| Studies | Place | Year of publication | Prevalence of enterococcal infection (%) |
|---|
| Phukan C et al., [13] | Assam | 2016 | 7.47 |
| Damrolien S et al., [8] | Manipur | 2018 | 1.01 |
| Present study | Manipur | 2021 | 1.03 |
The majority of the specimens were from inpatients (49%), which were correlated with the findings of Acharya A et al., reporting 72% of specimens from hospitalised patients and 28% from outpatients [14]. In this study, a total of 10.3% of enterococcal isolates were from ICU patients which was concordance with the findings of Tripathi A et al., (8.3%) and Paule SM et al., (13.9%) [15,16]. Patients who are admitted in ICU are at greatest risk of acquiring nosocomial infections, partly because of serious underlying disease, and also because of life saving invasive procedure, prolonged use of invasive devices and extended hospital stay. Among the ICUs, highest enterococcal infection was observed in SICU. The reason could be as post-surgical patients have longer hospital stay due to surgical site infection, soft tissue infection and have more chances of cross contamination [17].
Present study reflected E. faecalis as the predominant species (82.2%) followed by E. faecium (15.8%), E. durans (1.3%) and E. gallinarum (0.7%). Perlada ED et al., reported similar findings with 69% E. faecalis, 29% E. faecium, and 1% each of E. avium and E. durans [18]. This study demonstrated that E. faecalis (83.3%) as the predominant species isolated from urine samples and E. faecium (26%) in blood sample. However, Mohanty S et al., reported E. faecium (63%) as the most common isolate in blood [19].
A higher isolation rate of 69.2% was observed among the female patients than male (30.8%) which is comparable to that of Bose S et al., with females accounting for 80.4% and males 14.6% of cases [20]. Maximum number of isolates in females was seen in the age group of 21-30 years with a mean age of 33.59±18.29 years whereas in case of male, maximum isolates were found in 51-60 years with a mean age of 41.8±22.28 years. Moreover, E. faecalis was found predominant in 21-30 years age group whereas E. faecium in 31-40 years. Telkar A et al., found that, in 0-20 year age group, enterococcal isolates were highest in both the genders. However, Suchitra JB et al., found enterococci in the age group of 25 to 65 years with a mean age of 43.04±10.8 years [21,22].
On studying the antibiotic susceptibility pattern it was found that most of E. faecalis isolates were highly resistant to ampicillin (75%) than E. faecium isolates which was contradictory to the finding of Agarwal J et al., reporting significantly higher resistance E. faecium isolates to ampicillin than E. faecalis. The reason of such contrary could be due to the fact that more number of E. faecalis was isolated in our study. Enterococci are intrinsically resistant to most β-lactam antibiotics because of low affinity Penicillin Binding Proteins (PBP), which enable them to synthesise cell wall components [23]. The present study also demonstrated that enterococcal isolates were 73.9% resistance to ciprofloxacin, 34.4% to nitrofurantoin10.3% to vancomycin, 7.5% to teicoplanin and 3.4% to linezolid. Bhuyan B and Das PP reported 69.9% resistance to ciprofloxacin, 17% to nitrofurantoin, 6.6% to vancomycin and 0% to linezolid [24]. However, Zhanel GG et al., observed that none of the 300 isolates of enterococci tested were resistant to nitrofurantoin [25]. That is why, at present nitrofurantoin is used increasingly to treat nosocomial urinary tract infection caused by vancomycin resistant enterococci as it can be of utmost utility in cases of multidrug resistant strains of enterococci in urine.
Limitation(s)
In this study, minimum inhibitory concentration and genotyping of vancomycin resistance enterococci could not be performed due to lack of infrastructure.
Conclusion(s)
The present study highlights the occurrence of E. faecalis and E. faecium as the predominant enterococcal species in our health care set up. Enterococcal isolates were shown higher resistance to ampicillin, ciprofloxacin, nitrofurantoin and least to linezolid followed by teicoplanin and vancomycin. It is necessary to implement infection control measures like antimicrobial stewardship especially restricting the use of antibiotics to minimum.
Further research and progress for the vancomycin resistance enterococci are required to establish a more rational approach towards improving patient outcome.
*n=No. of isolates
*n=No. of isolates, MICU: Medicine intensive care unit; SICU: Surgical intensive care unit; PICU: Paediatric intensive care unit
n=No. of isolates
n=No. of isolates
*n=No. of isolate
Note: As, the number of isolates are not equal, all the antibiotics are not used for all isolates; n=no. of isolates, AMP: Ampicillin; CIP: Ciprofloxacin; VAN: Vancomycin; TEI: Teicoplanin; NIT: Nitrofurantoin; LZ: Linezolid; HLG: High level gentamycin; HLS: High level streptomycin; *For urine (n=116) samples and catheter tip (n=3): AMP, CIP, LZ, NIT, VAN AND TEI were used; *For Blood (n=13), pus (n=10), wound swab (n=4): AMP, VAN, TEI, LZ were used; *HLG and HLS were used for all isolates ; *For Intermediate isolates (n=19): Vancomycin, Teicoplanin, Nitrofurantoin