The IHCP is the most common of the four causes of raised liver enzymes unique to pregnancy. The incidence in India is 1.2 to 1.5% and varies widely all over the world [1]. It is characterised by pruritis without a rash that usually presents in the second and third trimester. Mutations of various genes, most commonly the ABCB4 gene, are found to impair the enterohepatic circulation which increases the level of bile acids in the serum to greater than 10 μmol/L [2]. The IHCP is associated with high concentrations of oestrogen which cause cholestasis and later on resolution of the disease postnatally, when oestrogen levels return to prepregnancy state with the delivery of the placenta [3].
Raised serum bile acids are the key diagnostic laboratory parameter in more than 90% cases. They are also accompanied by raised transaminases in 60% cases [4-8]. Both ALT and Aspartate Transaminase (AST) are markers of liver dysfunction and increase in their levels from just above normal levels to a few hundreds also supports the diagnosis of IHCP [9]. Viral hepatitis, use of hepatotoxic drugs, past history of liver/gall bladder diseases and other causes of raised transaminases need to be ruled out. Increased levels of ALT are better marker of liver damage than AST.
The pruritis in the mothers has a predilection for the palms and soles and is not accompanied by a rash. Clinical jaundice is rare and constitutional symptoms of cholestasis like anorexia and abdominal pain may be present. Foetal implications are far worse. Over the years, adverse foetal outcomes like preterm birth, meconium passage, intrapartum non reassuring Foetal Heart Rate (FHR), sudden Intrauterine Unexplained Death (IUD), Respiratory Distress Syndrome (RDS) and NICU admission have been found to be associated with IHCP [10-13].
Predicting IHCP well in time can prevent such foetal morbidity and mortality. Markers like serum bile acids and transaminases can guide management if correlated with outcomes. Serum bile acids >40 μmol/L had been associated with adverse foetal outcomes, while levels >100 μmol/L indicate increased risk for IUD [10,14]. Early term delivery is advocated, before a high and critical level of bile acids is reached. However, early delivery causes complications of prematurity in the newborn due to which pharmacological treatment was also tried to improve foetal prognosis. The first line drug is Ursodeoxycholic Acid (UDCA). Studies have found that UDCA mainly treats pruritis but has varying results with respect to benefit for perinatal outcome [15-18].
Increased bile acid levels are gold standard for the diagnosis of IHCP and critical levels have been defined for prediction of adverse perinatal outcome. However, bile acid testing is costly and not widely available in India, hence, transaminase levels are used instead for diagnosis and guiding management. Thus, the present study was proposed to find out the critical levels of ALT for prediction of adverse foetomaternal outcomes.
Materials and Methods
This was a case-control study carried out from October 2018 to March 2020, in the Obstetrics and Gynaecology Department of a tertiary care centre of North India. Before starting the study ethical approval was taken from the Institutional Ethical Committee, IEC/VMMC/SJH/Thesis/October/2018-218. After taking informed consent, 75 women with IHCP and 75 controls with matching demographic criteria were included in the study. The cases and controls were recruited from the antenatal clinic.
Inclusion criteria: Women with singleton pregnancies with confirmed gestational age (3rd trimester), cephalic presentation, having pruritis without a rash and deranged transaminases were included [9]. Controls were demographic and clinical criteria matched pregnant women without pruritus who attended antenatal clinic on the day of recruitment of cases.
Exclusion criteria: Women with ultrasound diagnosed obstructive causes of cholestasis, pruritis due to pre-existing skin disease, viral hepatitis, chronic liver disease or pregnancy specific causes of elevated liver enzymes such as pre-eclampsia, Haemolysis, Elevated Liver Enzymes, Low Platelet (HELLP) syndrome, acute fatty liver of pregnancy and women on drugs that effect Liver Function Tests (LFT) were excluded.
Sample size calculation: The sample size was calculated taking 76.47% sensitivity and 78.38% specificity of alanine aminotransferase level in predicting adverse perinatal outcomes at the cut-off value of 95 IU/L, as described by Ekiz A et al., [19]. Taking these values as reference, the minimum required sample size with desired precision of 15%, 80% power of study and 5% level of significance was 51.
Study Procedure
The ALT levels at diagnosis were noted for all the women. The normal values of ALT and AST in the third trimester were taken to be <35 U/L [5]. The women were followed-up as per institutional protocols. They were treated with UDCA 300 mg twice a day. Foetal well being was monitored by weekly Outpatient Department (OPD) visits for outpatients and Non-Stress Test (NST) biweekly for inpatients after a Period Of Gestation (POG) of 30 weeks. Termination of pregnancy was done by 37 weeks (or earlier if indicated) irrespective of the levels of transaminases in diagnosed patients of IHCP on UDCA, as per hospital protocol. The patients’ labour was monitored with a partogram and cardiotocography. Maternal and foetal outcomes were noted in both cases and controls. Testing for normalisation of LFTs was done 6 weeks postpartum.
The maternal outcomes measured were term/preterm delivery, onset of labour (spontaneous or induced), mode of delivery (normal/instrumental/caesarean section), Antepartum Haemorrhage (APH), Postpartum Haemorrhage (PPH), Intensive Care Unit (ICU) admission and maternal mortality. The foetal outcomes measured were preterm delivery, non-reassuring FHR, Meconium Stained Liquor (MSL), IUD, 5 minute APGAR score, NICU admission and RDS. If more than one adverse event was present in a woman, for the analysis of outcome each was taken as an independent event. For example, if a woman had MSL and prematurity, she was included in both categories.
Statistical Analysis
The data was entered in MS Excel spreadsheet and analysis was done using SPSS version 21.0. Quantitative variables were compared using Mann-Whitney Test. Qualitative variable were compared using Chi-Square test/Fisher’s-exact test. The cut-off of ALT for prediction of adverse maternal and foetal outcome was calculated from the receiver operating characteristic curve. The p-value of <0.05 was considered statistically significant.
Results
During the study duration of 18 months, a total of 3500 pregnant women attending antenatal clinic were screened. The women meeting inclusion and exclusion criteria were followed till outcome of consecutive 75 cases and 75 controls were obtained.
The cases and controls had a mean age of 24.81±4.2 years and 25.95±5.13 years, the mean age and parity of women in case and control group was comparable, p=0.274 and p=0.343, respectively. The patients were diagnosed between 28-39 weeks, with a mean gestation of 34±2.89 weeks. The IHCP group had significantly higher levels of mean ALT, 129.4±45.55 U/L (range 70-240 U/L) and mean AST, 113.49±43.84 U/L (range 66-250 U/L) as compared to controls, p-value <0.0001. The mean gestation at delivery amongst the cases was 37.49 weeks, significantly lower than the controls with 38.613 weeks (p<0.0001). A significantly higher number of cases of IHCP underwent induction of labour compared to controls, p=0.0003. Seven cases and four controls had a mild PPH and were managed medically. They didn’t require blood transfusion or surgical intervention [Table/Fig-1].
Comparison of maternal outcomes between cases and controls.
| Maternal parameters | Cases (N=75) | Controls (N=75) | p-value |
|---|
| Number (n) | Percentage % | Number (n) | Percentage % |
|---|
| Period of gestation at delivery (weeks)† |
| <37 | 5 | 6.67 | 3 | 4 | <0.0001 |
| 37+6 days | 37 | 49.33 | 8 | 10.67 |
| 38+6 days | 18 | 24 | 22 | 29.33 |
| 39+6 days | 13 | 17.33 | 19 | 25.33 |
| ≥40 | 2 | 2.67 | 23 | 30.67 |
| Type of labour‡ |
| Induced labour | 47 | 62.67 | 25 | 33.33 | 0.0003 |
| Spontaneous labour | 28 | 37.33 | 50 | 66.67 |
| Mode of delivery* |
| Caesareans delivery | 8 | 10.67 | 10 | 13.33 | 0.875 |
| Instrumental delivery | 3 | 4 | 2 | 2.67 |
| Normal vaginal delivery | 64 | 85.33 | 63 | 84 |
| APH** | 0 | 0 | 1 | 1.33 | 1 |
| PPH** | 7 | 9.33 | 4 | 5.33 | 0.533 |
| ICU admission | 0 | 0 | 0 | 0 | - |
| Maternal mortality | 0 | 0 | 0 | 0 | - |
†Chi-square test, 38.354, ‡Chi-square test, 12.927, *Chi-square test, 0.43, **Fisher-exact test; APH: Anti-partum haemorrhage; PPH: Post-partum haemorrhage; ICU: Intensive care unit
There was a significantly higher number of babies born with MSL in the IHCP group, p=0.002. All the babies born with low APGAR and those admitted to NICU were managed conservatively and discharged with a satisfactory condition [Table/Fig-2].
Comparison of foetal outcomes between cases and controls**.
| Foetal outcome | Cases (N=75) | Controls (N=75) | p-value |
|---|
| Number (n) | Percentage (%) | Number (n) | Percentage (%) |
|---|
| Prematurity | 5 | 6.67 | 3 | 4 | 0.719 |
| Non reassuring foetal heart rate | 7 | 9.33 | 4 | 5.33 | 0.533 |
| Meconium stained liquor | 18 | 24 | 4 | 5.33 | 0.002 |
| Intrauterine deaths | 3 | 4 | 1 | 1.33 | 0.62 |
| APGAR at 5 minutes | 6 | 8 | 2 | 2.67 | 0.276 |
| Neonatal ICU admission | 3 | 4 | 1 | 1.33 | 0.62 |
| Respiratory distress syndrome | 3 | 4 | 1 | 1.33 | 0.62 |
**Fisher-Exact test; APGAR: Appearance, pulse, grimace, activity, and respiration
The ALT levels associated significantly with the occurrence of MSL, IUD and NICU admissions. The MSL occurred at a mean ALT value of 149±49.65 U/L (p=0.041), IUDs occurring at a mean value of 215.33±5.03 U/L (p=0.01) and NICU admissions at a mean value of 219±7.21 U/L (p=0.006) [Table/Fig-3]. Serum ALT of 133 U/L at the time of diagnosis was predictive of adverse foetal outcomes, p=0.0019, the sensitivity, specificity and likelihood ratio of 65.71% (95% CI 47.8-80.9%), 82.5% (95% CI 67.2-92.7%) and 3.76, respectively [Table/Fig-4,5]. Also, ALT value of more than 133 U/L was associated with induction of labour and MSL, p=0.041 and 0.001, respectively [Table/Fig-6].
Association of ALT levels with adverse foetal outcomes†.
| Outcome | Range of ALT (U/L) | Mean±Std Deviation of ALT (U/L) | p-value |
|---|
| Preterm delivery (n=5) | 88-144 | 110±22.03 | 0.503 |
| Non reassuring FHR (n=7) | 70-225 | 135.43±60.59 | 0.949 |
| MSL (n=18) | 74-240 | 149±49.65 | 0.041 |
| IUD (n=3) | 210-220 | 215.33±5.03 | 0.01 |
| 5 minute APGAR ≤7 (n=6) | 70-225 | 158.17±66.74 | 0.3 |
| NICU admission (n=3) | 211-225 | 219±7.21 | 0.006 |
| RDS (n=3) | 70-225 | 123.67±87.81 | 0.343 |
†Mann-Whitney test; FHR: Foetal heart rate; IUD: Intrauterine unexplained death; NICU: Neonatal Intensive care unit; RDS: Respiratory distress syndrome; ALT: Alanine aminotransferase
Receiver operating characteristics curve of ALT (U/L) for predicting foetal adverse outcome.
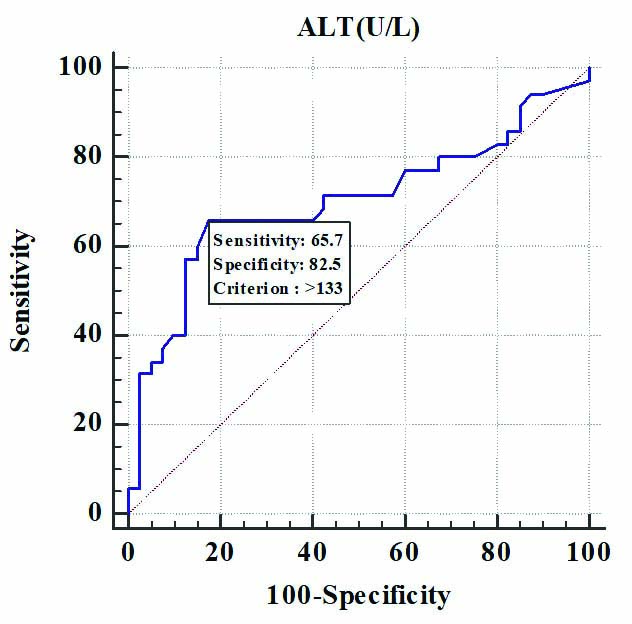
Sensitivity, specificity of ALT(U/L) for predicting foetal adverse outcome.
| ALT (U/L) | Preterm delivery | Non reassuring FHS | MSL | IUD | APGAR <7 | NICU | RDS | Perinatal adverse outcome |
|---|
| Area under the ROC curve (AUC) | 0.59 | 0.507 | 0.66 | 0.94 | 0.628 | 0.968 | 0.662 | 0.701 |
| Standard error | 0.093 | 0.158 | 0.0794 | 0.0278 | 0.182 | 0.0215 | 0.324 | 0.0649 |
| 95% CI† | 0.470 to 0.702 | 0.389 to 0.625 | 0.541-0.765 | 0.860-0.982 | 0.509-0.737 | 0.898-0.995 | 0.544-0.767 | 0.584-0.801 |
| p-value | 0.3333 | 0.963 | 0.0441 | <0.0001 | 0.4816 | <0.0001 | 0.6173 | 0.0019 |
| Cut-off | ≤113 | >80 | >133 | >202 | >176 | >210 | ≤76 | >133 |
| Sensitivity (95% CI) | 80% (28.4-99.5) | 57.14% (18.4-90.1) | 72.22% (46.5-90.3) | 100% (29.2-100.0) | 66.67% (22.3-95.7) | 100% (29.2-100.0) | 66.67% (9.4-99.2) | 65.71% (47.8-80.9) |
| Specificity (95% CI) | 54.29% (41.9-66.3) | 11.76% (5.2-21.9) | 70.18% (56.6-81.6) | 93.06% (84.5-97.7) | 85.51% (75.0-92.8) | 94.44% (86.4-98.5) | 94.44% (86.4-98.5) | 82.5% (67.2-92.7) |
| PPV (95% CI) | 11.1% (3.1-26.1) | 6.2% (1.7-15.2) | 43.3% (25.5-62.6) | 37.5% (8.5-75.5) | 28.6% (8.4-58.1) | 42.9% (9.9-81.6) | 33.3% (4.3-77.7) | 76.7% (57.7-90.1) |
| NPV (95% CI) | 97.4% (86.5-99.9) | 72.7% (39.0-94.0) | 88.9% (75.9-96.3) | 100% (94.6-100.0) | 96.7% (88.7-99.6) | 100% (94.7-100.0) | 98.6% (92.2-100.0) | 73.3% (58.1-85.4) |
CI: Confidence interval; PPV: Positive predictive value; NPV: Negative predictive value
Comparison of maternal and foetal outcomes between cases with ALT values above and below our predictive cut-off (133 U/L); Chi-square test.
| Maternal and foetal outcomes | ALT ≤133 (n=45) (%) | ALT >133 (n=30) (%) | Total (%) | p-value |
|---|
| Parity |
| Nulliparous | 19 (42.22) | 12 (40) | 31 (41.33) | 0.848 |
| Multiparous | 26 (57.78) | 18 (60) | 44 (58.67) |
| Gestation age at delivery |
| <34 weeks | 19 (42.22) | 10 (33.33) | 29 (38.67) | 0.326 |
| 34-37 weeks | 18 (40) | 10 (33.33) | 28 (37.33) |
| >37 weeks | 8 (17.78) | 10 (33.33) | 18 (24) |
| Onset of labour |
| Induced | 24 (53.33) | 23 (76.67) | 47 (62.67) | 0.041 |
| Spontaneous | 21 (46.67) | 7 (23.33) | 28 (37.33) |
| Mode of delivery |
| Caesarean delivery | 5 (11.11) | 3 (10) | 8 (10.67) | 0.672 |
| Instrumental delivery | 1 (2.22) | 2 (6.67) | 3 (4) |
| Normal vaginal delivery | 39 (86.67) | 25 (83.33) | 64 (85.33) |
| Complications |
| Antepartum haemorrhage | - | - | - | - |
| Postpartum haemorrhage |
| 3 (6.67) | 4 (13.33) | 7 (9.33) | 0.427 |
| Preterm delivery | 4 (8.89) | 1 (3.33) | 5 (6.67) | 0.642 |
| Non reassuring foetal heart rate | 3 (6.67) | 4 (13.33) | 7 (9.33) | 0.427 |
| Meconium stained liquor | 5 (11.11) | 13 (43.33) | 18 (24) | 0.001 |
| Intrauterine death | 0 (0) | 3 (10) | 3 (4) | 0.06 |
| 5 minute APGAR ≤7 | 2 (4.44) | 4 (13.33) | 6 (8) | 0.21 |
| NICU | 0 (0) | 3 (10) | 3 (4) | 0.06 |
| Respiratory distress syndrome | 2 (4.44) | 1 (3.33) | 3 (4) | 1 |
Discussion
The pruritis in IHCP can be distressing, leading to sleepless nights for the affected woman. However, the implications for the foetus are more worrisome. Sudden IUD is the most dreaded foetal outcome. Maternal bile acids cross the placenta and accumulate in the foetus and amniotic fluid causing sudden death due to foetal arrhythmias caused by vasospasm of placental chorionic vessels. Incidences of other related adverse outcomes like MSL, meconium aspiration syndrome and RDS also increase due to increased colonic motility caused by raised bile acids in amniotic fluid, leading to meconium passage [4,14,10,20]. The UDCA has been efficacious in alleviating maternal symptoms of pruritis and improving biochemical parameters, but its effect is controversial when it comes to preventing foetal morbidity and mortality. Thus, currently, the only way to prevent adverse foetal outcomes seems to be early termination before reaching critical high levels of bile acids, which keep on increasing with advancing gestational age.
The society for maternal foetal medicine advocate delivery at 36 weeks if total bile acid levels ≥100 μmol/L and at 36-39 weeks for patients with total bile acid levels <100 μmol/L (delivery at early end of this spectrum if bile acids ≥40 μmol/L) [21]. The American College of Obstetricians and Gynaecologists (ACOG) recommends delivery at 36-37 weeks, and RCOG calls for individualising management on the basis of severity of biochemical abnormalities while advocating offering induction to patients at 37 weeks [10,22].
Since, there was no facility for serum bile acid testing, the diagnoses was based on ALT and the pregnancies were terminated by 37 weeks. The present study aimed to find a cut-off of ALT which could guide management of these pregnancies like serum bile acids do, and achieve a balance between prevention of adverse outcomes and complications of prematurity.
Women with IHCP delivered at an early gestation compared to the controls. Labour was induced at 37 weeks in this group, as per institutional protocol irrespective of ALT levels. Similarly, studies by Geenes V et al., and Brouwers L et al., had IHCP mothers delivering significantly earlier [4,20]. But many were delivered iatrogenically even before 37 weeks, due to increased concerns with regard to risk of foetal death due to high maternal serum bile acids.
The IHCP women are at increased risk of PPH as coagulation function is affected secondary to vitamin K deficiency due to fat malabsorbtion because of the cholestasis. But occurrence of PPH was comparable amongst the cases and controls of this study. Dang A et al., however did find more PPH in their cases, but they did not exclude patients with co-morbidities like anaemia and gestational diabetes, which could have led to PPH on their own [23].
The number of vaginal, instrumental and caesarean deliveries was comparable between the cases and controls. Bile acids increase myometrial oxytocin receptors, due to which patients responded well to induction of labour and did not need caesareans. The finding of the present study were in accordance with the findings of Geenes V et al., and Kant A et al., but not with those of Dang A et al., who had more instrumental deliveries, likely due to inclusion of diabetic women [4,7,23].
Thus, women with IHCP suffered mainly from pruritis, and did not have greater morbidity related to operative deliveries and PPH as compared to controls. However, the foetal outcomes in this study cases were worse than the controls. A significantly higher incidence of meconium staining was found in the IHCP group in concordance with the results of many previous studies [4,7,23-25]. The meconium if aspirated can lead to non reassuring foetal heart rate, low APGAR scores and RDS which, however was not found to be significant in the present study, similar to previous studies [4,7,23-25].
Geenes V et al., found an association of stillbirth with increased bile acid levels. But in their study, 7 out of the 10 women with stillbirths had co-morbidities like pre-eclampsia which were excluded. We did not find increased stillbirths in present case group. Incidence of IUDs has progressively reduced to 3.5% or less in studies employing policies of active management with greater frequency of monitoring [8,10,11]. The incidence in the present study is a comparable 4% as the policy of early termination was followed. Most importantly, 3 patients in the study with IUD had irregular ANC follow-up and were admitted after 37 weeks, with documented foetal demise.
Transaminase levels were increased in most women with IHCP and their levels were unaffected by fasting or post-meal status, unlike bile acids, which were found to rise post-meals. Bile acids were also affected by gestational age while transaminase levels were not. A significant link between ALT and the occurrence of MSL, IUD and NICU admission was established. Previous studies didn’t find any such association of ALT with these outcomes per se, but they found ALT correlated with the occurrence of preterm delivery which was not always iatrogenic [4,19,26].
On comparing outcomes above and below the predictive cut-off of 133 U/L of ALT, it was concluded that MSL can be significantly predicted above this cut-off value and that induction of labour above these levels can be planned. Ekiz A et al., predicted the occurrence of spontaneous preterm delivery before 37 weeks above ALT levels >95 U/L [19]. More studies investigating the association of ALT with foetal outcomes in IHCP need to be conducted to evaluate these cut-offs. In present study, an indirect but cheap and commonly available marker with increased incidence of adverse outcomes in IHCP was evaluated. Most hospitals in India diagnose IHCP on the basis of LFT values, therefore finding a cut-off of ALT will help in optimising management of IHCP patients in low middle income countries.
Limitation(s)
Unavailability of serum bile acid testing, which would have helped to compare the sensitivity of ALT with that of bile acids so as to further evaluate the predictiveness of ALT.
Conclusion(s)
The IHCP babies had significantly worse outcomes in this study, which reinforces the need to be able to predict these adverse outcomes so as to prevent them. The occurrence of intrauterine demise in the patients who did not follow-up regularly establishes the need for regular OPD visits, monitoring of FHR and planned delivery. A cut-off of 133 U/L at the time of diagnosis has been found to be predictive of adverse foetal outcome like MSL. Management can be optimised by delaying delivery based on these transaminase levels and complications of prematurity due to universal policy of induced early term delivery can be avoided.
†Chi-square test, 38.354, ‡Chi-square test, 12.927, *Chi-square test, 0.43, **Fisher-exact test; APH: Anti-partum haemorrhage; PPH: Post-partum haemorrhage; ICU: Intensive care unit
**Fisher-Exact test; APGAR: Appearance, pulse, grimace, activity, and respiration
†Mann-Whitney test; FHR: Foetal heart rate; IUD: Intrauterine unexplained death; NICU: Neonatal Intensive care unit; RDS: Respiratory distress syndrome; ALT: Alanine aminotransferase
CI: Confidence interval; PPV: Positive predictive value; NPV: Negative predictive value