Pityriasis Versicolor, a commonly encountered superficial mycosis is a mild, chronic infection of the stratum corneum. Malassezia species are lipid dependent yeasts that are commonly found on human skin and associated with PV [1]. It is postulated that this disease occurs when the Malassezia species changes from yeast to mycelial form and then invade the skin [2]. This disease occurs worldwide with prevalence as high as 30-40% in populations in tropical regions, more frequently in areas with higher temperatures and higher relative humidity [3,4].
Dandruff is the most common, persistent, and relapsing scalp disorder that affects about half of the world population associated with Malassezia species and characterised by the formation of flaky white yellowish scales and itching of the scalp. The species that have been most strongly associated with dandruff are M.globosa, M.restricta, M.furfur, M.sympodialis, M.obtusa and M.slooffiae [5]. Besides the discomfort, this disorder is also socially embarrassing and affects the self-esteem of the patient [6,7].
Materials and Methods
This was a cross-sectional study carried out in the Department of Microbiology, MGIMS, Sevagram, Wardha, Maharashtra, India from January 2016 to July 2017. Study protocol was approved by Institutional Ethics Committee (IEC Approval No: MGIMS/IEC/MICR/101/2015 dated 23/11/2015) before initiation of the study. Dandruff severity was categorised as mild, moderate, and severe [5]. An informed consent of the patients was taken. Demographic data and detailed clinical history were recorded in a case proforma. Patients of all age groups were included in the study.
Inclusion criteria: The patients who attended Skin Out Patient Department (OPD) of tertiary care rural hospital were included. The clinically diagnosed patients of PV and dandruff with the symptoms of round to oval lesions either hypopigmented (white) or hyperpigmented (pink, tan, brown, black) were included in the study. In cases of dandruff, white to yellowish flakes were included.
Exclusion criteria: Patients who have already received anti-fungal agents and/or steroid treatment (topical or systemic) during last 2 weeks were excluded.
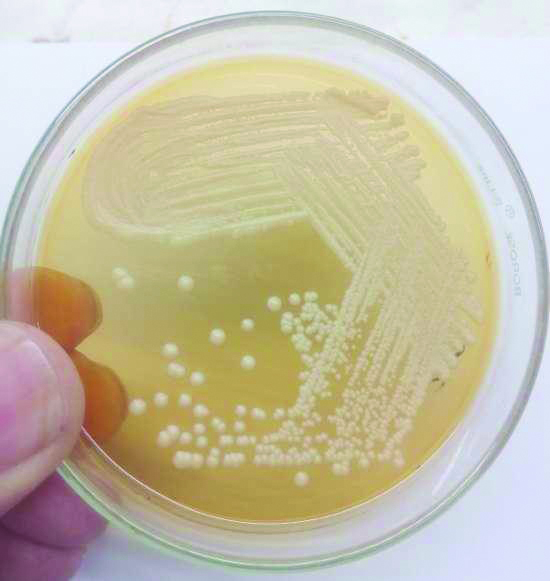
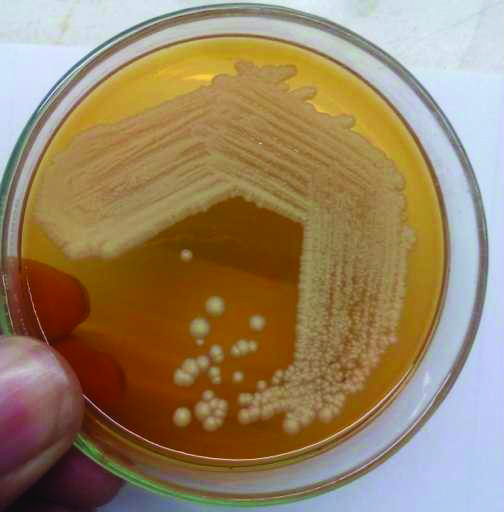
Skin scrapping were collected and processed for 10% KOH mount and culture on SDA, SDA overlaid with olive oil and mDA with antibiotics was done. Inoculated tubes were incubated at 32°C for four weeks [5]. Creamy white colonies of Malassezia were seen on SDA overlaid with olive oil and mDA [Table/Fig-1,2]. Speciation of Malassezia was done using catalase test, esculin hydrolysis and assimilation of Tween 20, 40, 60 and 80 [11].
Colonies of Malassezia on SDA overlaid with olive oil.
Colonies of Malassezia on modified Dixon agar.
Tween Assimilation Test
Utilisation of Tween compounds as a lipid source was evaluated. Yeast inoculum was prepared in 2 mL of sterile saline by picking five colonies. SDA melted in a boiling water bath and allowed to cool up to 47-48°C. Yeast suspension was then poured in to sterile petri dish and allowed to solidify at room temperature. Four holes were made and filled with 5 μL of Tween 20, 40, 60 and 80, respectively. The growth was assessed around the individual wells after two, four and seven days.
Catalase Test
Catalase test was done using 3% hydrogen peroxide. A drop of 3% hydrogen peroxide was poured on clean glass slide. A colony was picked up with glass rod and mixed. Immediate production of gas bubbles was considered as positive test.
Esculin Hydrolysis Test
Esculin agar slant was deeply inoculated with a loopful of yeast colony and incubated at 32°C for five days. Darkening of the slant indicates esculin hydrolysis. The test was used to distinguish M.furfur, M.slooffiae and M.sympodialis from other Malassezia species.
Statistical Analysis
In this study, as per SPSS version 16.0, the data was analysed using the Chi-squared test and p-value of <0.05 was considered statistically significant.
Results
A total of 178 cases of PV (127) and Dandruff (51) were included in the study. Out of 127 cases of PV, 71 (56%) were males and 56 (44%) were females. The highest number of patients were in the age group of 21-30 years [Table/Fig-3]. The details of the clinical history of cases of PV are given in [Table/Fig-4,5]. Among dandruff cases, 46 (90.19%) of patients were associated with application of oil in hair and 32 (62.75%) showed the soap usage and 19 (37.25%) showed shampoo as cleansing agent. Mild, moderate and severe dandruff was noted in 13 (25.49%), 31 (61%) and 7 (13.72%) cases, respectively. In 32 (63%) cases scalp hair were oily and 19 (37.25%) cases had dry scalp and hair fall was recorded in 29 (57%) patients.
Age wise distribution of cases among PV and dandruff.
| Age (Years) | PV n (%) | Dandruff n (%) |
|---|
| <10 | 4 (3.14) | 4 (7.84) |
| 11-20 | 25 (19.68) | 11 (21.56) |
| 21-30 | 52 (40.94) | 16 (31.37) |
| 31-40 | 26 (20.47) | 6 (11.76) |
| 41-50 | 13 (10.23) | 7 (13.72) |
| >50 | 7 (5.51) | 7 (13.72) |
Clinical history of cases in PV.
| Symptoms | Number of cases n (%) |
|---|
| Hypopigmented patches | 106 (83.46) |
| Hyperpigmented patches | 21 (16.53) |
| Itching | 53 (41.73) |
| No itching | 74 (58.26) |
Distribution of site of lesions in cases of PV.
| Site of lesion | Number of cases n (%) |
|---|
| Chest | 39 (30.71) |
| Neck | 26 (20.47) |
| Back | 24 (18.90) |
| Arm | 17 (13.39) |
| Face | 6 (4.72) |
| Multiple sites | 15 (11.81) |
Overall KOH positivity was 68/178 (38.20%) [Table/Fig-6]. KOH positivity was higher among dandruff patients than PV patients. Out of the 44 KOH positive PV cases, 37 (84.10%) showed characteristic Spaghetti and meatball appearance while 7 (15.90%) showed only budding yeast cells. However, in case of 24 KOH positive dandruff patients, all showed budding yeast cells only.
KOH and Culture positivity in cases of PV and Dandruff.
| Test | PV (n=127) | Dandruff (n=51) | Total (N=178) |
|---|
| KOH positive | 44 (34.64%) | 24 (47.05%) | 68 (38.20%) |
| Culture positive | 49 (38.58%) | 27 (52.94%) | 76 (42.69) |
On culture, Malassezia species were grown in all the patients showing Spaghetti and meatball appearance. Additionally, culture detected Malassezia species in four patients in which 10% KOH mount was negative.
Overall culture positivity was 76/178 (42.69%). In case of PV and dandruff, 38.58% and 52.94% yielded growth of Malassezia species respectively [Table/Fig-6]. No significant difference was found in Malassezia species distribution among PV and dandruff in study area (p>0.05) [Table/Fig-7].
Malassezia species distribution in cases of PV and Dandruff.
| Species | PV (n=49) | Dandruff (n=27) | Total (n=76) |
|---|
| M.sympodialis | 26 (53.06%) | 17 (62.96%) | 43 (56.58%) |
| M.furfur | 13 (26.53%) | 7 (25.93%) | 20 (26.32%) |
| M.globosa | 8 (16.33%) | 3 (11.11%) | 11 (14.47%) |
| M.slooffiae | 2 (4.08%) | 0 | 2 (2.63%) |
No significant difference found in Malassezia species distribution among PV and dandruff in study area (p>0.05)
Discussion
Yeasts of the genus Malassezia cause various skin lesions, among which PV and dandruff are the most common and worldwide in occurrence [2,3]. The various host factors can cause shift in the skin microbial flora associated with disease [12]. In the present study, the maximum patients affected were in the age group 21-30 years. Similar finding was reported by Romald PN et al., [13]. The chest, neck and back were the most involved sites. Similar findings were reported by Tarazooie B et al., and Rao GS et al., [11,14]. Majority of the cases were presented with hypopigmented lesions. Other studies from India, Ghosh SK et al., Archana BR et al., and Krishnan A and Thapa DM, also showed predominance of hypopigmented lesions in PV [15-17]. Overall KOH positivity was 38.20% (68/178). However, Kannan P et al., reported KOH positivity of 56.4% [18]. On culture, 76 (42.69%), Malassezia isolates were grown. Our culture results were comparable with Gupta AK et al., who had reported the similar isolation rates (43.8%) [19]. However, Romald PN et al., reported 70% rate of isolation of Malassezia in cases of PV [13].
In the present study, M.sympodialis was the predominant isolate followed by M.furfur, M.globosa and M.slooffiae in cases of PV. Global epidemiological data compiled by Rodoplu G et al., suggested that the most prominent species among PV patients in different countries are M.globosa followed by M.furfur and M.sympodialis [7]. Archana BR et al., Kindo AJ et al., from South India and Gupta AK et al., have reported M.sympodialis to be the predominant isolate followed by M.globosa and M.furfur [16,20,21]. However, Chaudhary R et al., from central India, Shah A et al., Dutta S et al., Kaur M et al., from North India [22-25], and Ghosh SK et al., have reported M.globosa to be the predominant isolate [15]. This may be due to varying geographical and environmental conditions.
Data on the epidemiology of dandruff in India is scarce [5,10]. In the present study, M.sympodialis, M.furfur and M.globosa were the predominantly isolated species. Previous study by Rudramurthy SM et al., had reported M.restricta and M.globosa as prevalent species in north India and M.furfur, M.restricta and M.globosa as main species in south India [5]. In a recent publication from north India, M.restricta, M.globosa along with M.furfur were common isolates [10]. However, Sumathi M and Growther L, had found M.sympodialis as prominent species [26] while another study by Kavitha K et al., reported M.furfur as most common species followed by M.restricta and M.slooffiae [27]. Occurrence and distribution pattern of different Malassezia species among dandruff scalp could be influenced by local geographical and environmental conditions, varying level of sebaceous secretion and individual sensitivity to a particular species [28].
Limitation(s)
Small sample size was a major limitation of the present study.
Conclusion(s)
M.sympodialis was most commonly associated with PV and dandruff followed by M.furfur and M.globosa in this rural area. Studies with larger sample size are needed to understand the relationship between these clinical conditions and the distinct species with an emphasis on their distribution.
No significant difference found in Malassezia species distribution among PV and dandruff in study area (p>0.05)