Heart Failure (HF) is a global health problem affecting an estimated over 37.7 million people worldwide [1]. In Sub-Saharan Africa (SSA) including Tanzania, HF is emerging as an important non-communicable disease which contributes significantly to morbidity and mortality [2-4]. In Tanzania, HF accounts for over 30% of admissions in cardiac and 18.5% in internal medicine wards [4,5]. Patients with HF normally require multiple drugs most of which are lifelong, therefore medications adherence is an important component of HF management [6]. Lack of medications adherence poses a significant challenge in achieving the targeted HF treatment goals [7], and has been reported to range between 40% and 60%, globally [8]. In SSA non-adherence to HF medications ranges from 26.2% to 74.7% as documented in published literature, depending on the study setting [9-13], and has been linked to poor outcome among HF patients [13].
The association between C-Reactive Protein (CRP) as an important marker of systemic inflammation underlying various cardiovascular diseases, including HF has been well established [14-17]. Serum concentration of CRP is elevated in patients with HF [16,18], and higher CRP levels are associated with more severe congestion [19]. More recently, the availability of hsCRP assays that are able to detect CRP levels as low as <0.3 mg/L have evolved [20], and have paved way for more research including assessment of efficacy of HF medications [21]. Several studies, mostly from Europe and North America have documented lowered hsCRP levels following use of HF medications among HF patients [19,22], and that measurements of hsCRP can predict medications adherence [23]. However, both levels of hsCRP and response to HF medications differ between ethnic groups [24,25], underscoring the need for local studies among native Africans. There is however paucity of research data relating hsCRP, HF and medications use among HF patients in SSA, including Tanzania. This study was aimed to assess the association between hsCRP levels and medication adherence status among HF patients attending a cardiac referral hospital in Tanzania.
Materials and Methods
This was a cross-sectional study conducted in the out-patient clinics of the Jakaya Kikwete Cardiac Institute (JKCI) in Dar es Salaam, Tanzania. JKCI is a National and University Teaching Cardiac Institute in Tanzania, receiving cardiac referral cases from all around Tanzania and from within Dar es Salaam. The study was conducted in accordance with the Helsinki Declaration of studies involving human subjects. Ethical approval to conduct the study was obtained from Muhimbili University of Health and Allied Sciences Research and Publication Committee (MUHAS Ref: Ref. No.MU/PGS/SAEC/Vol.IX/30). Each study participant freely signed an informed consent form before proceeding with data collection.
Inclusion criteria: All HF patients aged ≥18 years and who were on HF medications for atleast three months using any or all of the drugs including Angiotensin Converting Enzyme Inhibitors (ACEIs), Angiotensin Receptors Blockers (ARBs), aldosterone inhibitors, vasodilators, hydralazine, digoxin, diuretics and beta blockers were enrolled for the study.
Exclusion criteria: Patients who were on regular use of Aspirin, those who had taken antibiotics less than two weeks before the study as well as patients with documented current infection were excluded.
Sample size estimation: Based on the reported prevalence of medications non adherence of 40-60% [8], the population prevalence (p) was taken as 40% in order to obtain a maximum sample size (n), while setting the confidence level at 95%, (z=1.96) and a margin of error (e) of 7%. The sample size was calculated using the formula: n=z2p (100-p)/e2. Where, z=point of normal distribution corresponding to the 95% level of confidence (z=1.96). Substituting in the formula and adding 10% for non-response, a minimum sample size of 206 patients was required.
Data Collection Procedures
A research assistant screened and identified eligible patients, then invited them to take part in the study. A structured questionnaire was used to gather patients’ socio-demographic and clinical characteristics including their current medications use. Clinical information was also obtained from patients case records and information such as types of medications taken, underlying cardiac condition, co-morbidities and HF duration were recorded.
Patients were then given the 8-Item Morisky medications adherence questionnaire to fill in [26]. Assessment of medications adherence was done for all HF medications that the patient was prescribed. Patients who were unable to read and write were assisted in reading out the questions and their responses were filled in by the research assistant. The 8-item Morisky Medication Adherence Scale (MMAS-8) is a self-report questionnaire with 8 questions (items), which can give scores ranging from 0 to 8. The scores are categorised into three levels of adherence: high adherence (score=8), medium adherence (score of 6 to <8), and low adherence (score <6) [26]. Good adherence was considered as those with high scores, while medium and low adherence was considered as poor adherence, as previously reported [27].
All patients then underwent anthropometric and Blood Pressure (BP) measurements. Height and weight were measured and were used to calculate Body Mass Index (BMI) as weight (Kilogram) divided by height (metres2). Obesity was defined as BMI ≥30 kg/m2. Waist and hip circumference were measured using a tape measure. Waist circumference was measured at the level of the umbilicus and hip circumference at the level of the widest part of the hip. Waist circumference was considered increased when it was >102 cm in men and >88 cm in women [28]. BP and pulse rate were measured using electronic BP machine (Mindray® Patient Monitor; Shenzhen, China) and it was measured thrice, and the average of the last two measurements was taken as the patient’s BP. Hypertension was categorised as grade 1 (140-159/90-99 mmHg), grade 2 (160-179/100-109 mmHg) and grade 3 (≥180/≥110 mmHg) according to European Society of Cardiology guidelines [29].
Laboratory Procedures and Interpretation
For each patient, 5 mL of venous blood was taken and sent to the Muhimbili Central Pathology Laboratory for the analysis of hsCRP, Complete Blood Count (CBC) and Cholesterol panel.
Blood sample for hsCRP was collected into heparinised tubes. At the laboratory, the samples were centrifuged to get the plasma. The analysis of hsCRP was done by validated automated Enzyme-Linked Immunosorbent Assay (ELISA) nephelometry, which uses particle-enhanced immunonephelometry technique to quantify hsCRP in serum samples. Samples were automatically diluted at 1:10 by the ARCHITECT® Systems machine prior to analysis. Polystyrene particles coated with monoclonal antibodies against hsCRP became agglutinated when mixed with samples containing hsCRP. This agglutination (measured by nephelometer) is detected as an absorbency change (572 nm), (intensity of light scattering due to the agglutination reaction) with rate of change being proportional to the quantity of hsCRP in a sample. The assay was standardized against the reference material for plasma protein preparation, CRM 470 [30]. hsCRP was considered elevated when it was >5 mg/L, according to the Muhimbili Central Pathology Laboratory reference range as well as from previous literature [17].
Blood was also analysed for CBC at the same laboratory. Important parameters analysed were haemoglobin concentration, white blood cell count, platelet count, neutrophil count, and lymphocyte count. Parameters for blood cholesterol analysed were total blood cholesterol, Low Density Lipoprotein Cholesterol (LDL-C) and High Density Lipoprotein Cholesterol (HDL-C). Anaemia was defined as haemoglobin concentration of <13 g/dL in men and <12 g/dL in women. Total cholesterol was considered elevated when it was above 5.2 mmol/L and LDL-C was considered elevated when it was above 3.36 mmol/L. White blood cells count was considered raised when it was >9.8×109/L, in accordance with the Laboratory’s reference values.
Statistical Analysis
Data were analysed using SPSS computer software, version 21. Univariate analysis using mean and median as a measure of central tendency, and range and standard deviation as measures of dispersion were employed for quantitative variables. Proportion(s) were applied for categorical data such as adherence status, sex and type of HF. Comparison of two means was carried out by Student’s t-test. Chi-square was employed for testing statistical significance for frequency distribution of categorical data. Uni- and multivariate logistic regression analyses were used to determine the independent associations of poor adherence. The results were of statistical significance when p-value was <0.05.
Results
A total of 500 participants were approached and screened to be included in the study. Of these, 176 patients were not eligible, 40 patients declined to participate while 35 withdrew from the study during the data collection process. Of the remaining 249 patients, blood test results of 39 patients could not be retrieved from the laboratory leaving 210 available for the final analysis.
Socio-demographic characteristics of participants: [Table/Fig-1] summarises the socio-demographic characteristics of the study population. The mean age of the total population was 54±15.9 years (range 19-95), and 113 (53.8%) were females. Half (50.0%) of participants were older adults aged above 55 years. Also, half (50.0%) of the participants had attained primary education. Half of the study participants were insured, while the remaining half were not insured therefore buying their own medications. The mean duration from the diagnosis of HF was 3.3 years (range 1-36 years), and hypertension was the most common cardiovascular risk factor being present in 147 (70%) of the total study population [Table/Fig-1].
Socio-demographic characteristics of the study population.
| Characteristics | Frequency (N=210) | Percent (%) |
|---|
| Age group (years) |
| ≤35 | 32 | 15.2 |
| 36-55 | 73 | 34.8 |
| ≤56 | 105 | 50 |
| Sex |
| Male | 97 | 46.2 |
| Female | 113 | 53.8 |
| Level of education |
| No formal education | 31 | 14.8 |
| Primary education | 105 | 50 |
| Secondary education | 55 | 26.2 |
| College/University | 19 | 9 |
| Occupation |
| Not employed/peasant | 103 | 49 |
| Employed/business | 80 | 38.1 |
| Retired | 27 | 12.9 |
| Marital status |
| Single | 12 | 5.7 |
| Married | 142 | 67.6 |
| Divorced | 5 | 2.4 |
| Widowed | 36 | 17.1 |
| Separated | 15 | 7.2 |
| Hospital payment mode |
| Insured | 105 | 50 |
| Public/Exempted/Private | 105 | 50 |
| Cardiovascular risk factors |
| Hypertension | 147 | 70 |
| Diabetes mellitus | 21 | 10 |
| Alcohol consumption | 65 | 31 |
| Smoking | 34 | 16.2 |
Anthropometric, Blood Pressure (BP) and clinical findings of participants: Anthropometric and BP findings are shown in [Table/Fig-2]. The mean Body Mass Index (BMI) of the total population was 26.7±6.2 kg/m2 and obesity was present in 41 (19.5%) participants. Majority of participants (81%) had higher than the recommended waist/hip ratio as per WHO criteria, while elevated waist circumference was present in 73 (34.8%) participants. In the total population, stage 1, stage 2 and stage 3, hypertension was present in 24.8%, 11.4% and 7.6% of patients, respectively. Co-morbidities and medications used by study participants are shown in [Table/Fig-3].
Anthropometric and Blood Pressure (BP) findings.
| Variables studied | Values |
|---|
| Height (cm) (Mean±SD) | 159.7±8.5 |
| Weight (kg) (Mean±SD) | 68.1±16.3 |
| Body mass index (kg/m2) (Mean±SD) | 26.7±6.2 |
| Obesity status, n (%) |
| Normal weight n (%) | 86 (41) |
| Overweight n (%) | 83 (39.5) |
| Obese n (%) | 41 (19.5) |
| Waist circumference (cm) (Mean±SD) | 90±14 |
| Hip circumference (cm) (Mean±SD) | 98±14 |
| Waist to hip ratio (cm) (Mean±SD) | 0.92±0.04 |
| Proportion with elevated waist/hip ratio, n (%) | 170 (81) |
| Proportion with elevated waist circumference, n (%) | 73 (34.8) |
| Pulse rate (beats/min) (Mean±SD) | 84±18 |
| Systolic blood pressure (mmHg) (Mean±SD) | 128±32 |
| Diastolic blood pressure (mmHg) (Mean±SD) | 80±21 |
| Hypertension stage, n (%) |
| Normal blood pressure n (%) | 118 (56.2) |
| Stage 1 n (%) | 52 (24.8) |
| Stage 2 n (%) | 24 (11.4) |
| Stage 3 n (%) | 16 (7.6) |
Co-morbidities and medications used by study participants.
| Co-morbidities | Number (n) | Percentage (%) |
|---|
| Hypertensive heart disease | 126 | 60 |
| Dilated cardiomyopathy | 73 | 34.8 |
| Rheumatic heart disease | 6 | 2.8 |
| Others | 5 | 2.4 |
| Medications used |
| Diuretics | 184 | 87.6 |
| Beta-blockers | 163 | 77.6 |
| ACEI/ARB | 161 | 76.7 |
| Nitrates | 51 | 24.3 |
| Calcium channel blocker | 34 | 16.1 |
| Digoxin | 23 | 11 |
| Statins | 19 | 9 |
| Hydralazine | 13 | 6.2 |
ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blocker
Laboratory findings: [Table/Fig-4] summarises the laboratory findings in the total population. Anaemia was present in 113 (53.8%) patients, while 107 (51%) and 12 (5.7%) patients were found to have elevated total cholesterol and raised white blood cell count, respectively. The mean hsCRP in the total study population was 7.1±4.9 mg/L (range 0.2 to 31.0 mg/L). The median (Interquartile range) was 5.8 mg/L (5.3 mg/L). Elevated hsCRP was present in 122 (58.1%) patients.
Laboratory findings in the total study population.
| Laboratory findings | Mean±SD/n (%) |
|---|
| Haemoglobin (g/dL) (Mean±SD) | 12.2±1.5 |
| Proportion with anaemia, n (%) | 113 (53.8) |
| Total cholesterol (mmol/L) (Mean±SD) | 5.1±1.2 |
| Proportion with elevated total cholesterol, n (%) | 107 (51) |
| LDL-cholesterol (mmol/L) (Mean±SD) | 3.5±1.02 |
| Proportion with elevated LDL-cholesterol, n (%) | 114 (54.3) |
| HDL-cholesterol (mmol/L) (Mean±SD) | 1.12±0.39 |
| Proportion with low HDL-cholesterol, n (%) | 95 (45.2) |
| WBC count (×109/μL) (Mean±SD) | 6.8±2.13 |
| Proportion with elevated WBC count, n (%) | 12 (5.7) |
| hsCRP (mg/L) (Mean±SD) | 7.1±4.9 |
| Proportion with elevated hsCRP levels, n (%) | 122 (58.1) |
LDL: Low density lipoprotein cholesterol; HDL: High density lipoprotein; WBC: White blood cell; hs-CRP: High-sensitivity C-reactive protein
Adherence to HF medications: In the Morisky medications adherence tool, 72 (34.3%) patients scored high, 101 (48.1%) scored medium, while 37 (17.6%) scored low. Accordingly, 72 (34.3%) patients who scored high were categorised as having good adherence and 138 (65.7%) who scored medium and low were categorised as having poor adherence. Patients with poor HF medications adherence did not differ from those with good adherence in terms of sex distribution, age, marital status, level of education, employment status or whether they were insured or not, all p>0.05 [Table/Fig-5]. They also did not differ in all clinical and anthropometric parameters including smoking status, obesity and BP levels, all p>0.05 [Table/Fig-5].
Socio-demographic and clinical characteristics of patients in relation to HF medications adherence status.
| Characteristic | Good adherence (n=72) | Poor adherence (n=138) | p-value |
|---|
| Female sex, n (%) | 41 (56.9) | 72 (52.2) | 0.510 |
| Age (years) (Mean±SD) | 54.2±13.4 | 54.2±17.1 | 0.995 |
| Age >55 years, n (%) | 35 (48.6) | 70 (50.7) | 0.771 |
| Living without partner, n (%) | 24 (33.3) | 44 (31.9) | 0.831 |
| Below secondary education, n (%) | 47 (65.3) | 89 (64.5) | 0.910 |
| Employment status, n (%) |
| Unemployed | 37 (51.4) | 66 (47.8) | 0.884 |
| Employed/business | 26 (36.1) | 54 (39.1) |
| Retired | 9 (12.5) | 18 (13.0) |
| Not insured, n (%) | 38 (52.8) | 67 (48.6) | 0.561 |
| Taking alcohol, n (%) | 24 (33.3) | 41 (29.7) | 0.590 |
| Smokers, n (%) | 11 (15.3) | 23 (16.7) | 0.795 |
| Admitted previous year, n (%) | 28 (38.9) | 45 (32.6) | 0.364 |
| Obese, n (%) | 17 (23.6) | 24 (17.4) | 0.280 |
| Central obesity, n (%) | 25 (34.7) | 48 (34.8) | 0.993 |
| Systolic BP (mmHg) (Mean±SD) | 126±30 | 129±34 | 0.485 |
| Diastolic BP (mmHg) (Mean±SD) | 79±18 | 81±22 | 0.510 |
| ≤Stage 2 hypertension, n (%) | 10 (13.9) | 30 (21.7) | 0.169 |
BP: Blood pressure; Categorical variables compared by Chi-square test; Differences between means compared by Student’s t-test; *p-value <0.05 to be considered significant
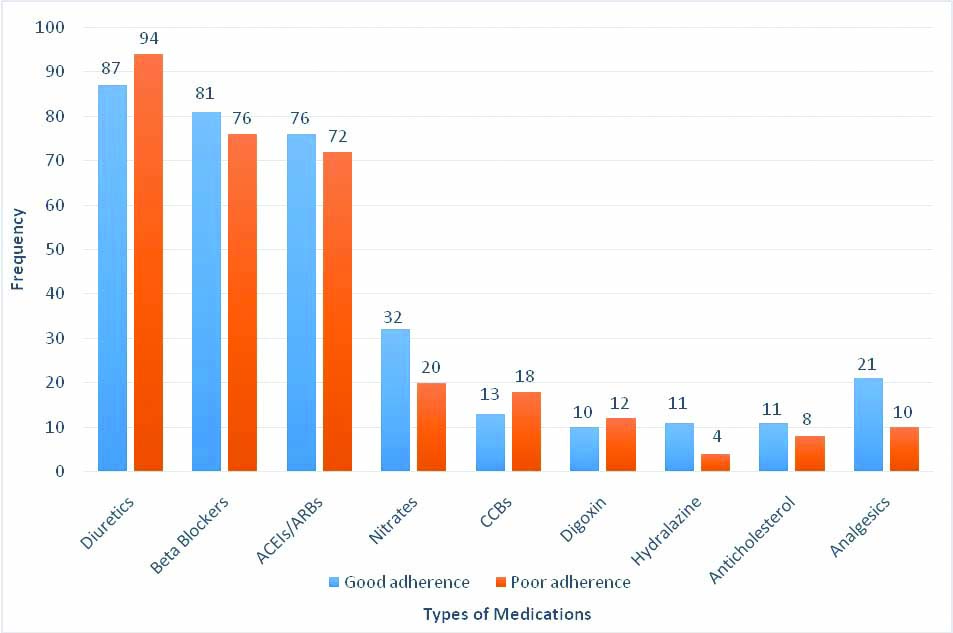
[Table/Fig-6] shows medications used and their frequency in relation to HF medications adherence status of the patients. Patients with poor adherence were less likely to use hydralazine as well as analgesics. There was however no difference between the two groups in terms of the use of diuretics, beta blockers, ACEIs/ARBs, nitrates, calcium channel blockers, digoxin or anticholesterol medications, all p>0.05.
HF medications use in relation to adherence status.
ACEIs: Angiotensin enzyme inhibitors; ARBs: Angiotensin receptor blockers; CCBs: Calcium channel blockers.*Analgesics other than aspirin. Categorical variables compared by Chi-square test
[Table/Fig-7] compares laboratory findings in relation to medications adherence status. Patients with poor adherence had significantly higher mean hsCRP levels (7.75±5.00 mg/L) when compared to those with good adherence (5.72±4.59 mg/L), p=0.004. Consequently, the proportion of patients with elevated hsCRP was significantly higher in the group of patients with poor adherence to HF medications. The mean WBC count did not differ between patients with poor adherence compared to those with good adherence, and neither did the proportion of patients with elevated WBC count. The mean total cholesterol level and mean HDL-cholesterol was significantly higher in the group of patients with poor adherence, both p<0.05.
Laboratory findings in relation to HF medications adherence status.
| Laboratory finding | Good adherence (n=72) | Poor adherence (n=138) | p-value |
|---|
| hsCRP (mg/L) (Mean±SD) | 5.72±4.59 | 7.75±5.00 | 0.004 |
| Elevated hsCRP, n (%) | 28 (38.9) | 94 (68.1) | <0.001 |
| Haemoglobin (g/dL) (Mean±SD) | 12.1±1.3 | 12.2±1.7 | 0.902 |
| Anaemia, n (%) | 36 (50.0) | 77 (55.8) | 0.424 |
| WBC count (x109/L) (Mean±SD) | 6.7±1.8 | 6.8±2.2 | 0.640 |
| Raised WBC count, n (%) | 2 (2.8) | 10 (7.2) | 0.185 |
| Platelets count (x109/L) (Mean±SD) | 233±70 | 242±77 | 0.423 |
| Elevated platelets count, n (%) | 1 (1.4) | 3 (2.2) | 0.693 |
| Low platelet count, n (%) | 9 (12.5) | 11 (8.0) | 0.289 |
| Total cholesterol (mmol/L) (Mean±SD) | 4.8±1.3 | 5.2±1.2 | 0.031 |
| Elevated total cholesterol, n (%) | 30 (41.7) | 77 (55.8) | 0.052 |
| LDL-cholesterol (mmol/L) (Mean±SD) | 3.32±1.02 | 3.58±1.01 | 0.072 |
| Elevated LDL-cholesterol, n (%) | 32 (44.4) | 82 (59.4) | 0.039 |
| HDL-cholesterol (mmol/L) (Mean±SD) | 0.99±0.36 | 1.18±0.39 | 0.001 |
| Low HDL-cholesterol, n (%) | 42 (58.3) | 53 (38.4) | 0.006 |
hsCRP: High sensitivity C-reactive protein; WBC: White blood cell count; LDL: Low density lipoprotein; HDL: High density lipoprotein; Categorical variables compared by Chi-square test; Differences between means compared by Student’s t-test
Factors found to have atleast weak associations (p<0.5) with poor medications adherence were entered into a multivariate logistic regression analysis in order to determine the independent associations of having poor HF medications adherence. Factors entered into the model included obesity, diabetes mellitus, admission in the past 1 year, having ≥ stage 2 hypertension on the day of recruitment, elevated hsCRP, anaemia, as well as elevated WBC count and total cholesterol. In addition, all medications found to have had associations with medications adherence were included in the model. Sex and age were lastly entered into the regression analysis as important confounders [Table/Fig-8].
Independent predictors of poor HF medications adherence obtained in multivariate logistic regression analysis.
| Variable | Univariate analysis | Multivariate analysis |
|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value |
|---|
| Female sex | 0.83 (0.47-1.46) | 0.511 | 0.95 (0.45-2.01) | 0.902 |
| Age >55 years | 1.09 (0.62-1.92) | 0.771 | 1.56 (0.75-3.27) | 0.239 |
| Obesity | 0.68 (0.34-1.37) | 0.282 | 0.61 (0.25-1.48) | 0.273 |
| Diabetes mellitus | 0.43 (0.18-1.08) | 0.071 | 0.43 (0.14-1.29) | 0.128 |
| Admitted last year | 0.76 (0.42-1.38) | 0.365 | 0.80 (0.40-1.60) | 0.530 |
| ≤Stage 2 hypertension | 1.72 (0.79-3.76) | 0.172 | 2.72 (1.01-7.46) | 0.050 |
| Elevated hsCRP | 3.36 (1.85-6.08) | <0.001 | 4.27 (2.14-8.51) | <0.001 |
| Anaemia | 1.26 (0.71-2.24) | 0.424 | 1.50 (0.76-2.94) | 0.241 |
| Elevated WBC count | 2.73 (0.58-12.83) | 0.202 | 2.24 (0.41-12.37) | 0.354 |
| Elevated total cholesterol | 1.77 (0.99-3.15) | 0.053 | 1.53 (0.79-2.97) | 0.204 |
| ACEI/ARB use | 0.79 (0.41-1.52) | 0.470 | 0.88 (0.41-1.90) | 0.752 |
| Analgesics use | 0.43 (0.19-0.95) | 0.036 | 0.36 (0.14-0.92) | 0.033 |
| Nitrates use | 0.54 (0.28-1.04) | 0.063 | 0.51 (0.24-1.09) | 0.082 |
| Hydralazine use | 0.30 (0.10-0.96) | 0.042 | 0.09 (0.02-0.40) | 0.002 |
hsCRP: High sensitivity C-reactive protein; WBC: White blood cell count; ACEIs: Angiotensin enzyme inhibitors; ARBs: Angiotensin receptor blockers
Having elevated hsCRP was found to be strongly and significantly associated with poor medications adherence in univariate analysis and remained to be independently and even more strongly associated with poor medications adherence in the multivariate analysis, p<0.001. Furthermore, having ≥ stage 2 hypertension on the day of clinic visit became independently associated with poor medications adherence. Of note, elevated WBC (a measure of infection) did not predict poor adherence, and importantly did not alter the association between hsCRP and medications adherence status [Table/Fig-8].
Discussion
This study was done to determine the association between levels of hsCRP and medications adherence status among HF patients attending care and treatment at a tertiary health facility in Dar es Salaam, Tanzania. The main findings of the study are 3-fold: First, the prevalence of poor medications adherence among HF patients in this population is high (65.7%); second, the proportion of HF patients with elevated levels of hsCRP is also high (58.1%) and third, elevated hsCRP levels are strongly and independently associated with poor medications adherence in this population.
Poor adherence to medications among HF patients is a known problem worldwide and previous literature indicates the problem to range from 40% to 60% [8]. The prevalence of poor medications adherence found in this study is therefore almost similar to that reported in the previous studies. However, the prevalence reported in the present study is different from that reported at Chris Hani Baragwanath Hospital in Soweto, South Africa in which researchers examined the pattern of treatment adherence, self-care and treatment knowledge in 200 patients with chronic HF [11]. In the later study, the prevalence of poor HF medications adherence was 29%, much lower than the findings in the present study. The difference between the index study and that from South Africa is most likely due to the fact that the South African study was part of an on-going HF registry and it is possible that patients in that study population better adhered to their medications because they knew they were actively been followed-up. The 65.7% prevalence found in this study is however much lower than that reported at the cardiology clinic in Abidjan, Ivory Coast which was 88.4% [31]. In that study, the investigators used HF compliance questionnaire, and pointed out too much use of traditional medicine among HF patients as a factor that influenced poor medications adherence in that population [31].
Both the mean level of hsCRP (7.1±4.95 mg/L) and the proportion of HF patients with elevated hsCRP (58.1%) were high in the present study. Our findings confirm previous findings in documented literature and adds to the existing knowledge on the fact that patients with HF have an existing and on-going low level of inflammation, evidenced by increased levels of hsCRP in these patients [15,19]. The prevalence of elevated hsCRP found in the present study is remarkably similar to that found in big registries of HF patients following acute myocardial infarction in the United States of America; the TRIUMPH (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status) and the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) registries [32]. The registries included 3,410 patients and the prevalence of elevated hsCRP was reported to be 58.6%. The prevalence of elevated hsCRP levels in this study is however slightly higher than the 50% prevalence reported by the Val-HeFT (Valsartan in HF Trial), which involved 4,202 HF patients participating in a multinational randomised clinical trial to assess the efficacy of the Angiotensin receptor blocker Valsartan versus placebo in patients with NYHA classes 2-4 [19].
There is paucity of documented literature on hsCRP among HF patients in SSA, and only one previous study has been reported from the region [33]. In a study by Sliwa K et al., from South Africa, the reported median hsCRP was 10 mg/L (range 1-90 mg/L), and 45% of patients had elevated levels of hsCRP [33], with the differences most likely due to different definitions of elevated hsCRP used in the two studies. The finding that having BP readings equivalent to stage 2 or more hypertension (systolic BP ≥160 mmHg, and/or diastolic BP ≥100 mmHg) on the day of clinic visit is independently associated with poor medications adherence is an interesting one. Patients not adhering to their prescribed HF medications are also likely not to adhere to other chronically used medications, including those for BP control. This finding is not unique as has been reported by previous researchers [34,35], and is a recognised barrier to optimal care in patients with multiple drug use [36]. The finding is however very clinically important as control of hypertension among patients with HF is equally important due to the progressive nature of HF when co-morbid conditions, including hypertension are not controlled. Medications adherence is a potentially modifiable behaviour that should be monitored in patients taking long term medications [37]. It is only through efficient use of medications that the benefits of new therapies and advances in HF management will make an impact on the individual patient’s outcome.
This study found that patients who were using hydralazine and analgesics were less likely to be non-adherent to their overall HF medications. This finding is difficult to explain; it is however hypothesised that patients using analgesics are more likely to adhere to their medications due to the immediate effect of analgesics in alleviating pain. Further studies on patients’ behaviours towards specific drugs in relation to overall medications adherence are warranted.
A number of socio-demographic, economical and behavioural factors are known to influence medications adherence in patients on long term use of prescribed medications [34-37]. In the present study, none of the socio-demographic factors studied (age, sex, marital status, level of education, employment status, insurance status, alcohol use and cigarette smoking) was associated with medications adherence, contrary to findings in previous literature [28,34,35,37]. Since, the present study was primarily designed to determine the association between serum hsCRP levels and HF medications adherence, it was therefore not adequately powered to study the socio-demographic or behavioural determinants of poor medications adherence in this population. Further studies using appropriate sample size are recommended in order to ascertain these associations; if any, in our local setting.
Limitation(s)
The limitations of this study include its cross-sectional nature, and therefore causality cannot be confirmed. However, in the present study, it is almost only clinically plausible to say that hsCRP levels were raised following non-adherent to HF medications and not vice versa. Other factors that affect hsCRP levels were not systematically studied and it is therefore possible that some patients with good medications adherence continued to have elevated hsCRP due to other factors including improperly prescribed HF medications by attending physician, disease progression, etc. In addition, the Morisky medications adherence tool used in this study has its own limitations, which include a recall bias among patients.
Conclusion(s)
The prevalence of poor medications adherence is high among HF patients attending care and treatment at JKCI, which is a cardiac referral hospital in Tanzania. Poor adherence to HF medications was present in almost two-thirds of the population, and more than half of the patients had elevated levels of hsCRP. Furthermore, this study has found that elevated hsCRP is independently associated with poor HF medications adherence among HF patients attending care and treatment at JKCI. Further studies from different clinical settings are recommended to confirm these interesting findings obtained from this study. If confirmed, hsCRP level can, in the future be considered as a surrogate marker of HF medications adherence among HF patients in the local setting.
ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blocker
LDL: Low density lipoprotein cholesterol; HDL: High density lipoprotein; WBC: White blood cell; hs-CRP: High-sensitivity C-reactive protein
BP: Blood pressure; Categorical variables compared by Chi-square test; Differences between means compared by Student’s t-test; *p-value <0.05 to be considered significant
hsCRP: High sensitivity C-reactive protein; WBC: White blood cell count; LDL: Low density lipoprotein; HDL: High density lipoprotein; Categorical variables compared by Chi-square test; Differences between means compared by Student’s t-test
hsCRP: High sensitivity C-reactive protein; WBC: White blood cell count; ACEIs: Angiotensin enzyme inhibitors; ARBs: Angiotensin receptor blockers