Chronic Lymphocytic Leukaemia Presenting with Pancytopenia: A Rare Haematological Coincidence
Iffat Jamal1, Shuchi Smita2, Ravi Bhushan Raman3, Kaushal Kumar4, Vijayanand Choudhary5
1 Assistant Professor, Department of Haematology (Pathology), Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India.
2 Assistant Professor, Department of Haematology (Pathology), Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India.
3 Assistant Professor, Department of Haematology (Pathology), Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India.
4 Assistant Professor, Department of Haematology (Pathology), Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India.
5 Professor, Department of Haematology (Pathology), Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Iffat Jamal, Flat No. D/2, Phase-1, Sapna Apartment, Nayatola, Patna-800004, Bihar, India.
E-mail: iffatjamal111@gmail.com
Chronic Lymphocytic Leukaemia (CLL) is commonly associated with autoimmune cytopenias but CLL presenting with pancytopenia is a rare occurrence. Here, we present a case of 56-year-old male patient, who presented with pallor, mild hepatosplenomegaly, right-sided pleural effusion in chest X-ray, pancytopenia and his bone marrow showed infiltration by mature appearing lymphoid cells making a diagnosis of CLL. Despite the frequency of these immune mediated cytopenia, only few cases of pancytopenia have been described so far to the best of our knowledge. Hence, to emphasise the rarity of such haematological coincidence we are presenting this case report. CLL generally presents with persistent absolute lymphocytosis on peripheral smear with an absolute monoclonal lymphocyte count of more than 5000/cumm. There is a common association with Autoimmune Haemolytic Anaemia (AIHA) and Immune Thrombocytopenia (ITP) and the association is called as Evan’s syndrome. Its pathogenesis is associated with autoimmune process. AIHA, ITP, and Pure Red Cell Aplasia (PRCA) are commonly associated complications seen in CLL. The pathogenesis of AIHA and ITP are antibody this inplace of the reactions and abnormal T cell activity is noted in the pathogenesis of PRCA. Despite of the frequency of these immune mediated cytopenias noted in CLL, bone marrow was seen to be hypercellular in most of the cases and marrow hypoplasia have been reported in only 2 cases reported in literature so far. In the present case, although pancytopenia was present on peripheral blood smear examination but bone marrow was hypercellular for age with diffuse infiltration by monoclonal mature appearing lymphoid cells which makes this case all the more interesting. Despite repeated blood transfusions, antiviral drug Tenofovir and aggressive supportive measures for 6 months, he developed right-sided pleural effusion and succumbed to his illness.
Autoimmune, Cytopenias, Evans syndrome, Lymphocyte count
Case Report
A 56-year-old male patient presented with low grade fever, pain abdomen and weakness for five months with history of repeated blood transfusions in the past for low haemoglobin level. On clinical examination, he had pallor and mild hepatosplenomegaly. His chest X-ray revealed right-sided pleural effusion. Ultrasonography abdomen showed thickened and hypoechoic mural stratification of bowel loops with involvement of ileo-caecal junction and rectum with tortuous mesenteric vessels likely consistent with inflammatory changes. Few enlarged mesenteric lymph nodes were seen with size varying from 0.5 cm to 1.5 cm. Colonoscopy revealed pancolitis and Inflammatory bowel disease in diameter. However, colonoscopic biopsy could not be performed. Liver function tests were deranged with raised Alkaline phosphatase (201 IU/L) and reversed A:G ratio of 0.39. His serum Lactate dehydrogenase was 540 U/L.
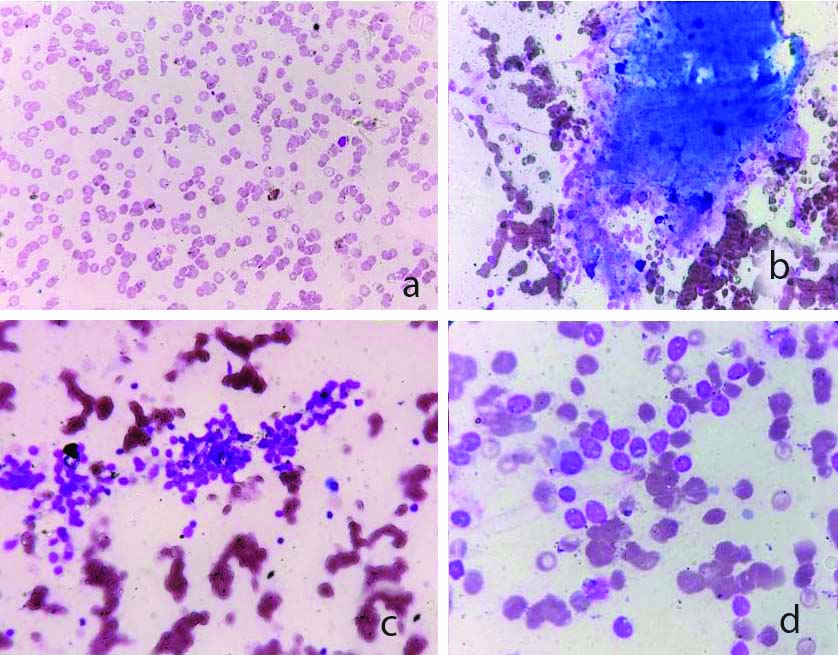
His complete blood count revealed pancytopenia with Haemoglobin of 5.1 gm/dl, RBC count of 1.66 millions/cumm and total WBC count of 1920/cumm. Differential WBC count was -Neutrophils-38%, lymphocytes-56%, Monocytes-5%, Eosinophils-1% and Basophils-0%. Peripheral blood smear showed microcytic hypochromic RBCs, leucopenia with relative lymphocytosis and thrombocytopenia. Reticulocyte count was 0.2%. Patient was subjected to bone marrow examination for evalualtion of persistent pancytopenia not responding to haematinics. Vitamin B12 and folate assays were normal but Serum iron and ferritin were low. As patient had pancytopenia on peripheral smear with low haemoglobin not responding to haematinics and blood transfusions, bone marrow aspiration was performed from right Posterior Superior Iliac Spine (PSIS) to rule out any haematological malignancy. Bone marrow aspirate showed a hypercellular marrow with infiltration by 88% mature looking lymphocytes and few plasmacytoid lymphocytes. At places lymphoid follicle formation also noted. There was marked suppression of trilineage haematopoiesis along with presence of many smudge cells in the background [Table/Fig-1a-d].
a) Peripheral blood smear showing pancytopenia (Leishman stain; X1000); b) Bone marrow aspirate showing hypercellular particle comprising of mature lymphoid cell clusters (Leishman stain; X100); c) Bone marrow aspirate showing mature lymphocyte clusters (Leishman; X400); d) Bone marrow aspirate showing mature lymphocyte clusters with trilineage suppression (Leishman; X1000).
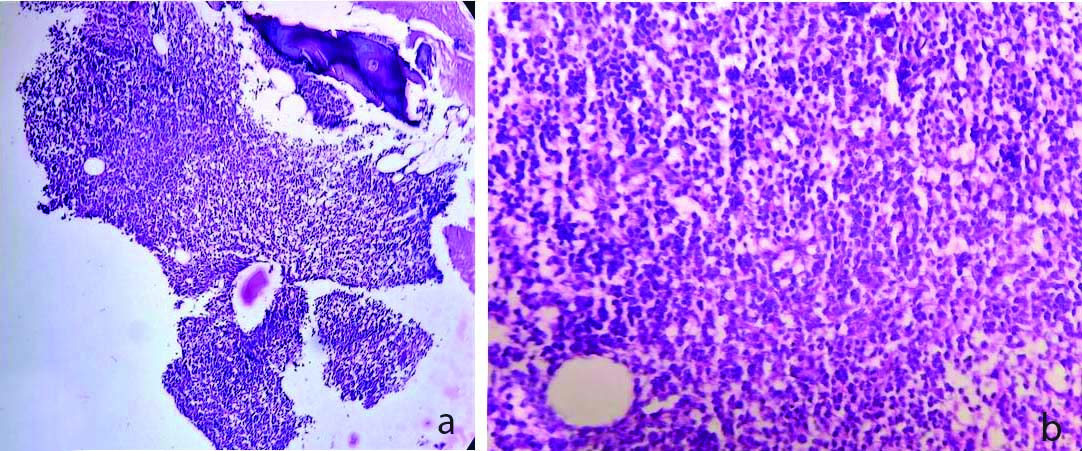
Bone marrow biopsy revealed diffuse infiltration of mature lymphoid cells along with few plasma cells consistent with diagnosis of Chronic lymphoproliferative disorder [Table/Fig-2a,b]. In order to rule out causes of reactive lymphocytosis and to establish clonality, Immunohistochemistry (IHC) was advised [Table/Fig-3a,b].
a) Bone marrow biopsy showing hypercellular marrow with diffusely arranged mature lymphocytes (H&E; X100); b) Bone marrow biopsy showing hypercellular marrow with diffuse infiltration by mature lymphocytes (H&E; X400).
a-c) IHC showing positivity for CD20, CD23, CD5 (DAB; X 400); d) IHC showing nuclear positivity for ki67 approximately 20% proliferative activity (DAB; X100).
IHC: Immunohistochemistry; CD: Cluster of differentiation antigen; DAB: 3,3′-Diaminobenzidine staining
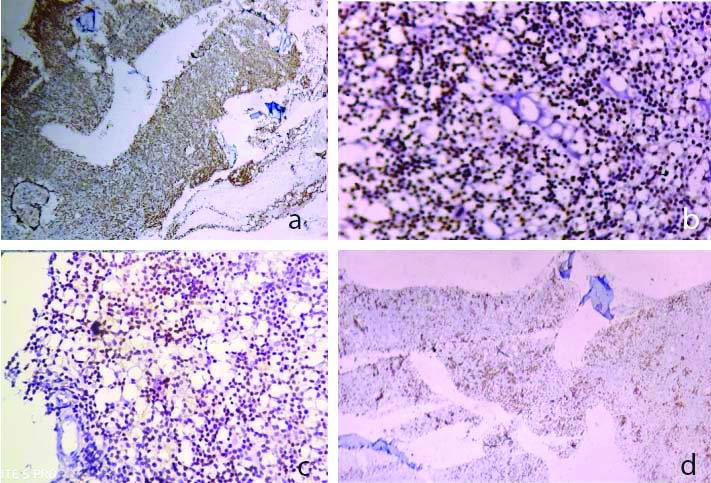
IHC revealed diffuse CD20, CD23 and focal CD5 positivity and expression of cytoplasmic lambda light chain. B-cell lymphoma 2 (bcl2) was also positive with Ki67 around 20% [Table/Fig-3a-d]. CD3, CD10 and cyclin D were negative. A diagnosis of CLL was rendered based on morphology and IHC. He was Hepatitis B surface Antigen (HBsAg) positive for which he was taking antiviral drug Tenofovir for the past 6 months. Despite repeated blood transfusions and aggressive supportive measures, he developed right-sided pleural effusion and succumbed to his illness. No autopsy was performed.
Discussion
CLL is known to be associated with autoimmune diseases including most commonly haematological conditions AIHA, ITP and PRCA and others being grave’s disease, myasthenia gravis etc. AIHA has been reported in one third of CLL patients [1,2]. Prevalence of ITP in CLL is 2% and PRCA is much less commonly seen [1,2].
In the present case, there were two distinct causes of pathogenesis of pancytopenia- one was heavy bone marrow infiltration by malignant clone of lymphoid cells and the other was T-cell dysfunctioning precipitated by infection with hepatitis B and use of Tenofovir, a purine analogues for Hepatitis B infection. These drugs have permissive effects on autoantibody formation by selective loss of a particular sub-population of T cells [3,4]. It has been observed that in PRCA and aplastic anaemia, there is reversal of C4/CD8 ratio, resulting in suppression of erythropoiesis via increased secretion of gamma-interferon [5]. Presentation with pancytopenia in the present case is quite rare and suggests T-cell dysfunctioning leading to trilineage suppression of haematopoiesis [6].
Earlier three cases of aplastic anaemia associated with CLL have been described so far. First case was a 58-year-old man developing PRCA few years after being diagnosed with B-CLL who then became pancytopenic with hypoplastic marrow with paratrabecular lymphoid aggregates which were CD19+, CD20+, CD23+, CD5+, and FITC- conjugated Mouse Monoclonal antibody (FMC7) with expression of cytoplasmic lambda light chains, consistent with a diagnosis of CLL. He was on treatment with Cyclosporine 6 mL/kg/day [7]. Second patient was a 42-year-old man with B-CLL that progressed over time and developed AIHA after starting low dose Chlorambucil (5 mg/day) and finally became pancytopenic with hypocellular bone marrow [8]. In the third case, a 71-year-old patient who was presented with pancytopenia, with a markedly hypocellular bone marrow and with prominent trabecular lymphoid follicles [9]. IHC revealed positivity for CD19, CD23, CD5 monoclonal lambda light chains leading to the diagnosis of CLL [9]. However, in the present case no chemotherapy was started at the time of diagnosis and that could be the major reason for the hypercellularity of bone marrow as anticancer drugs are well known for causing bone marrow suppression. In previous cases, they developed pancytopenia and aplastic bone marrow picture years after start of immunosuppressive agents and anticancer drugs [10-12]. Hepatitis B causes suppression of haematopoiesis and alters the natural history and aggressiveness of CLL [13]. On follow-up chemotherapy was started. Initially, his blood counts started to improve on start of chemotherapy but then he developed severe pancytopenia and succumbed to his illness. Treatment with chemotherapeutic agents and purine analogue could also be one of the causes of pancytopenia in this case.
In another report, 3 out of 100 patients suspected to have aplastic anaemia as initial presentation had low grade Non-hodgkin lymphoma, although no further details on the subtype of lymphoma had been described [14]. With development of secondary cytopenias in CLL, severity of disease progresses and prognosis worsens. Early detection of complications can help in providing effective management [15].
Conclusion(s)
There is only handful of cases where CLL is associated with pancytopenia which makes the present case worth reporting for timely diagnosis and treatment. Prognosis of such presentation is bad and requires immediate medical intervention. Association of Hepatitis B infection as in the present case and its treatment with purine analogue could be one of the cause of presentation with pancytopenia in CLL patients. So a proper medical history and history of medication in such patients is a must along a good clinician-pathologist communication.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 31, 2020
Manual Googling: Jan 22, 2021
iThenticate Software: Apr 16, 2021 (8%)
[1]. Visco C, Ruggeri M, Laura Evangelista M, Stasi R, Zanotti R, Giaretta I, Impact of immune thrombocytopenia on the clinical course of chronic lymphocytic LeukaemiaBlood 2008 111(3):1110-11.10.1182/blood-2007-09-11149217986663 [Google Scholar] [CrossRef] [PubMed]
[2]. Mauro FR, Foa R, Cerretti R, Giannarelli D, Coluzzi S, Mandelli F, Autoimmune haemolytic anemia in chronic lymphocytic Leukaemia: Clinical, therapeutic, and prognostic featuresBlood 2000 95(9):2786-92.10.1182/blood.V95.9.2786.009k30_2786_279210779422 [Google Scholar] [CrossRef] [PubMed]
[3]. Kyasa MJ, Parrish RS, Schichman SA, Zent CS, Autoimmune cytopenia does not predict poor prognosis in chronic lymphocytic Leukaemia/small lymphocytic lymphomaAm J Hematol 2003 74(1):01-08.10.1002/ajh.1036912949883 [Google Scholar] [CrossRef] [PubMed]
[4]. Kyasa MJ, Hazlett L, Parrish RS, Schichman SA, Zent CS, Veterans with chronic lymphocytic Leukaemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of deathLeuk Lymphoma 2004 45(4):507-13.10.1080/1042819031000161293915160912 [Google Scholar] [CrossRef] [PubMed]
[5]. Dearden C, Wade R, Else M, Richards S, Milligan D, Hamblin T, The prognostic significance of a positive direct antiglobulin test in chronic lymphocytic Leukaemia: A beneficial effect of the combination of fludarabine and cyclophosphamide on the incidence of hemolyticanemiaBlood 2008 111(4):1820-26.10.1182/blood-2007-07-10130318055869 [Google Scholar] [CrossRef] [PubMed]
[6]. Jaffe E, Harris N, Stein H, Vardiman J, Tumours of haematopoietic and lymphoid tissues 2001 4(2)IARC Press:127-30. [Google Scholar]
[7]. JA Zonder, Keating M, Schiffer CA, Chronic lymphocytic leukaemia presenting in association with Aplastic anemiaAmerican journal of Hematology 2002 71:323-27.10.1002/ajh.1022612447965 [Google Scholar] [CrossRef] [PubMed]
[8]. Clive SZ, Neil EK, Autoimmune complications in chronic lymphocytic leukaemiaBest Practice Res Clin Haematology 2010 23(1):47-59.10.1016/j.beha.2010.01.00420620970 [Google Scholar] [CrossRef] [PubMed]
[9]. Julia MB, Bhargava P, Medical mystery: A 71 year old man with pancytopenia-The answerNew England Journal of Medicine 2009 :2845-46.10.1056/NEJMc08647219109583 [Google Scholar] [CrossRef] [PubMed]
[10]. Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic LeukaemiaN Engl J Med 2003 348(4):1764-75.10.1056/NEJMoa02314312724482 [Google Scholar] [CrossRef] [PubMed]
[11]. Ibrahim S, Keating M, Do KA, O’Brien S, Huh YO, Jilani I, CD38 expression as an important prognostic factor in B-cell chronic lymphocytic LeukaemiaBlood 2001 98(5):181-86.10.1182/blood.V98.1.18111418478 [Google Scholar] [CrossRef] [PubMed]
[12]. Zent CS, Call TG, Hogan WJ, Shanafelt TD, Kay NE, Update on risk-stratified management for chronic lymphocytic LeukaemiaLeuk Lymphoma 2006 47(8):1738-46.10.1080/1042819060063403617064983 [Google Scholar] [CrossRef] [PubMed]
[13]. Keating MJ, O’brien S, Albitar M, Lerner S, Plunkett W, Giles F, Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic LeukaemiaJ Clin Oncol 2005 23(3):4079-88.10.1200/JCO.2005.12.05115767648 [Google Scholar] [CrossRef] [PubMed]
[14]. Byrd JC, Rai K, Peterson BL, Appelbaum FR, Morrison VA, Kolitz JE, Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic Leukaemia: An updated retrospective comparative analysis of CALGB 9712 and CALGB 9011Blood 2005 105(6):49-53.10.1182/blood-2004-03-079615138165 [Google Scholar] [CrossRef] [PubMed]
[15]. Gupta N, Kavuru S, Patel D, Janson D, Driscoll N, Ahmed S, Rituximab-based chemotherapy for steroid-refractory autoimmune hemolyticanemia of chronic lymphocytic LeukaemiaLeukaemia 2002 16(1):2092-95.10.1038/sj.leu.240267612357362 [Google Scholar] [CrossRef] [PubMed]