Quantitative Assessment of Interleukin-6 and Ferritin Levels and its Clinical Correlation among COVID-19 Patients
Susanna Theophilus Yesupatham1, Preethi Rathnasabapathy2, MP Sujatha3, SM Azeem Mohiyuddin4, Ravishankar Suryanarayana5
1 Associate Professor, Department of Biochemistry, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India.
2 Postgraduate Student, Department of Anaesthesia, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India.
3 Associate Professor, Department of Anaesthesia, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India.
4 Professor, Department of Otorhinolaryngology and Head and Neck Surgery, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India.
5 Assistant Professor, Department of Community Medicine, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. MP Sujatha, Associate Professor, Department of Anaesthesiology, Sri Devaraj Urs Academy of Higher Education and Research, Tamaka, Kolar-563103, Karnataka, India.
E-mail: susanna020682@gmail.com
Introduction
COVID-19 pandemic has emerged as a global challenge threatening human life worldwide. Early recognition of severe forms of COVID-19 infection is critically essential for timely triaging of COVID-19 patients. Biochemical Parameters correlating clinically with the severity of COVID-19 infection amidst testing of Peripheral Oxygen Saturation (SpO2) levels can serve in timely management of severe COVID-19 infections.
Aim
To estimate the concentrations of proinflammatory cytokine serum Interleukin-6 (IL-6) and serum ferritin levels and to clinically correlate these markers with COVID-19 disease severity.
Materials and Methods
A cross-sectional single center study conducted in a tertiary care hospital and research center from July 2020 to September 2020, 113 COVID-19 positive patients confirmed by Real Time-Polymerase Chain Reaction (RT-PCR) were included, Serum IL-6 and Serum Ferritin levels were measured in the patient’s blood sample using standard Enzyme linked Immunosorbent Assay (ELISA) and Vitros ECi Immunodiagnostics respectively. Data obtained was statistically analysed using Statistical Package for the Social Science (SPSS) Software version 22.0.
Results
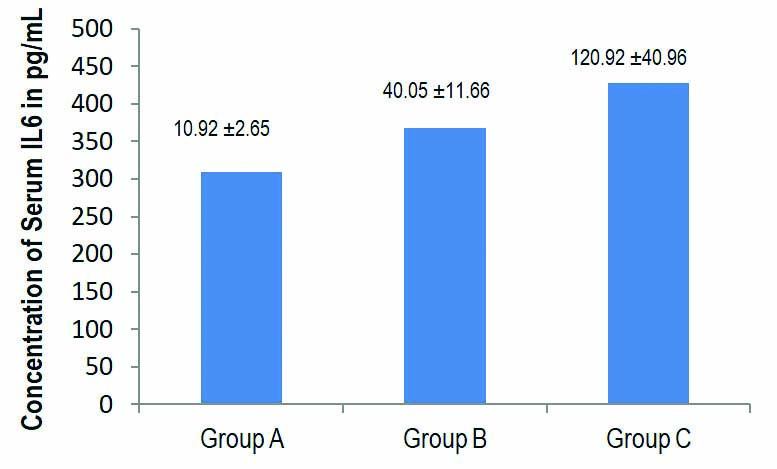
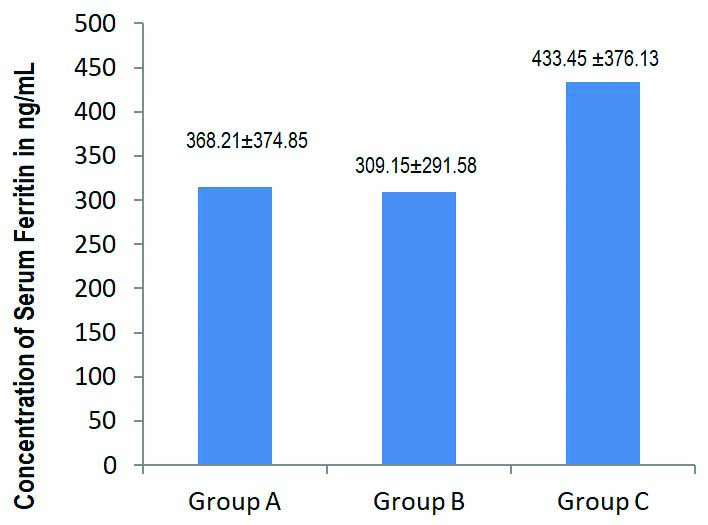
Of the 113 COVID-19 infected patients, 53 were included in Group A, 27 patients in Group B and 33 patients in Group C. Mean±SD of Serum IL-6 levels were 10.92±2.65, 40.05±11.66 and 120.92±40.96 pg/mL (p<0.001) and Serum Ferritin levels 368.21±374.85, 309.15±291.58, and 433.45±376.13 ng/mL, respectively.
Conclusion
IL-6 correlated significantly with disease severity of COVID-19 infection and can be judiciously used for stratification and management of COVID-19 infected patients. Serum Ferritin concentration were found to be high in severe cases of COVID-19 infections and did not show any significant variation compared with mild to moderate COVID-19 infection.
Acute phase protein, Proinflammatory cytokine, Severe acute respiratory syndrome coronavirus-2
Introduction
The Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection now popularly known as the COVID-19 or novel Coronavirus infection was declared a pandemic in March 2020. Since then there has been a dramatic increase in the number of SARS-CoV-2 infected patients globally [1]. Several cases with clinical presentation resembling viral pneumonia emerged with severe manifestations requiring treatment in Intensive Care Units (ICUs). The early recognition of severe forms of infection due to COVID-19 is essential to triage patients who might require treatment in these cases [2].
SARS-CoV-2 is a RNA virus belonging to the family coronaviridae, with the viral spike protein having 20-30 amino acids longer compared to other closely related coronaviruses. Apart from the Immune evasion of the host brought about by this family of viruses, the variation in the viral spike is hypothesised to give this strain an extra edge to facilitate the additional unidentified mechanisms by which the SARS-CoV-2 virus alters the pathogenesis of the disease particularly its severity and outcome [3].
Recent studies done in various countries have observed the development of severe manifestations of COVID-19 infection to be related to the hyperinflamatory state or cytokine storm. Cytokine storm secondary to hyperactivity of host immune responses and a massive inflammatory cell infiltration in the lungs causes Acute Respiratory Distress Syndrome (ARDS) [4,5]. Though the diagnosis of a COVID-19 infection is based on the presence of the virus in oropharyngeal swabs identified by RT-PCR, there is still a search for laboratory markers for early identification and stratification of patients, into those who might require intensive care therapy, immunosuppressant therapy and antiviral therapy.
According to the reports from Wuhan, China, where the first cluster of pneumonia cases due COVID-19 was reported and from other provinces [4-8], the severity of the COVID-19 infection has been related to the highly elevated inflammatory markers in the serum like ferritin, monocytes/macrophage activation, elevated pro inflammatory cytokine and chemokine levels like IL-1, IL-6 and Tumor Necrosis Factor-α (TNF-α) [9-12].
Among the biomarkers, increased Serum Ferritin and IL-6 levels have been suggested as prognostic indicators in COVID-19 patients [1]. Considering the fact that these biomarkers were tested during the early phase of the pandemic and most of the studies were retrospective in design and there may have been a change in the virulence of the COVID-19 infection due to human to human dissemination [13], this study aimed at estimating the concentration of IL-6 and Ferritin in the serum of the patients who were tested positive for COVID-19 infections and admitted at our hospital, and further correlating these levels with clinical presentation and severity of the disease due to COVID-19 infection.
Materials and Methods
This cross-sectional analytical study was conducted in a tertiary care hospital and research center attached to Sri Devaraj Urs Medical College, Kolar, Karnataka, India. The study was conducted between July 2020 to September 2020. Ethical clearance was obtained from Institutional Ethical Committee (IEC) Ref No: DMCKLR/IEC/306/2020-21dated 9-10-2020. Informed consent was obtained from all the participants.
Sample size calculation: Sample Size of 113 cases was estimated using n’master 2.0 sample size calculating software considering the IL-6 estimates as reported in a study by Chen X et al., variance estimate σ=40.96, at confidence interval of 95% and absolute precision of 8 pg/mL. The IL-6 levels in severe cases was (120.92±40.96). The total sample size was estimated to be 101+11 (non compliance)=112 patients. So, total 113 COVID-19 positive patients were taken for the study [14,15].
Inclusion criteria: All COVID-19 positive patients, diagnosed upon admission by RT-PCR of oropharyngeal swabs with or without respiratory symptoms were included in the study group.
Exclusion criteria: Patients younger than 18 years, critically ill patients like acute myocardial infarction patients during hospitalisation, diabetes mellitus with acute complications, acute pancreatitis, chronic kidney disease.
A total of 113 RT-PCR positive for COVID-19 virus were enrolled in the study and were segregated into three groups- Group A included asymptomatic patients and patients with mild symptoms with Respiratory Rate (RR) <24/m and SpO2 >94% in room air, Group B included symptomatic patients with mild to moderate pneumonia with RR: 24-30/m (or) SpO2: 90%-94% at Room Air, Group C included symptomatic patient with severe pneumonia with ARDS RR >30/min (or) SpO2 <90% at Room Air (or) less than 94% with Oxygen, ARDS. This categorising of patients was based on the new clinical management protocol: COVID-19 guidelines by Government of India, Ministry of Health and Family Welfare, Directorate General of Health services. Version 5.2020 [16]. A detailed clinical history of co-morbidities like diabetes mellitus, cardiac disease, tuberculosis, bronchial asthma and arthritis if any were recorded.
Method of Sample Collection
A 3 mL venous blood was collected from the patients with aseptic precautions, centrifuged at 3000 rpm for 5 minutes and the separated serum was used for Serum IL-6 estimation using Standard Enzyme Linked Immunosorbent Assay kits (Krishgen Bio Systems, India), as per kit instructions. Serum IL-6 levels were expressed in pg/mL.
Serum ferritin levels were estimated using automated clinical biochemistry analyser (Vitros ECi immunodiagnostic systems, Ortho clinical diagnostics, United Kingdom). Serum Ferritin levels expressed in ng/mL.
Statistical Analysis
Collected data was coded into excel format. Continuous data was presented with mean, standard deviation and confidence interval. The data was checked for normality and Mann-Whitney U test, and Kruskal Wallis Test was used to test significance of difference between the groups mean. A p-value <0.05 was considered as statistically significant. IBM SPSS 22 version software was used for data analysis.
Results
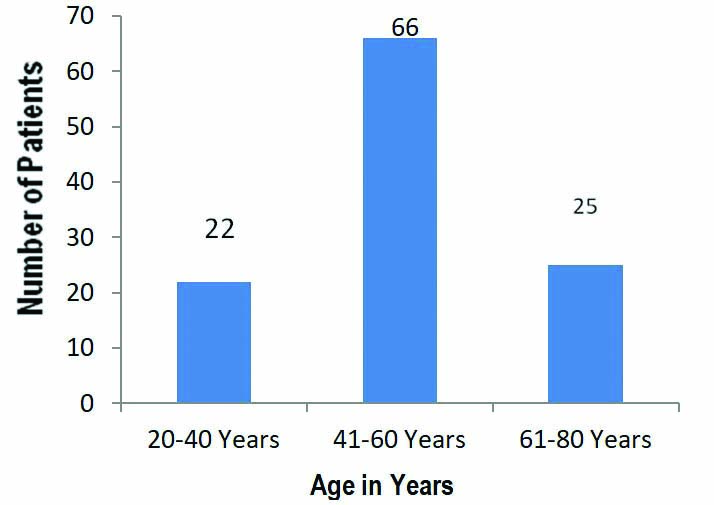
In the present study, 113 COVID-19 infected patients confirmed by RT-PCR were included. A total of 22 patients were aged between 20-40 years, 66 patients between 41-60 years age group and 25 patients were in 61-80 years of age group [Table/Fig-1]. The number of male patients was 76 and female patients were 37. Group A had 53 patients among which 6 patients were asymptomatic and 47 had mild symptoms with RR of <24/m and SpO2 of >94% in room air, the number of patients with co-morbidities like type 2 diabetes mellitus were 13, with hypertension and cardiac disorders were 11 and with respiratory disorders were 4 in Group A. In Group B there were 27 patients among which 7 had mild symptoms and 20 patients had moderate symptoms with RR: 24-30/m (or) SpO2 of 90%-94% at room air.
The number of COVID-19 positive patients according to age groups, most of the patients belonged to the age group between 41-60 years.
The mean levels with standard deviation of Serum IL-6 and serum ferritin in Group A, Group B and Group C patients, respectively has depicted in [Table/Fig-2,3]. On pairwise comparison by Mann-Whitney U test among the groups it shows that the mean IL-6 levels are significantly different in all the three groups. Kruskal Wallis pairwise comparison shows IL-6 levels to be significantly increased in Group C patients with severe disease compared to Group A and Group B patients with mild and moderate disease respectively (p-value <0.001). Serum Ferritin levels did not show any statistically significant difference among Group A, Group B and Group C, however ferritin levels were markedly increased in Group C patients with severe disease (p-value=0.428) [Table/Fig-4].
Bar Diagram showing Serum IL-6 Levels in pg/mL, expressed as mean difference and standard deviation in Group A, Group B, Group C with COVID-19 infection, respectively.
Bar diagram showing Serum Ferritin levels in pg/mL, expressed as mean difference and standard deviation in Group A, Group B, Group C with Mild, Moderate and Severe COVID-19 infection, respectively.
Correlation analysis of Serum IL-6 Levels and Serum Ferritin levels in Group A, Group B and Group C patients with Mild, Moderate and Severe disease of COVID-19 infection; Mann-Whitney U test and Kruskal Wallis pairwise comparison. Serum IL-6 was an indicator of COVID-19 disease severity (p<0.001 ***highly significant) compared to Serum Ferritin.
| Parameters | N | Mean | Std. Deviation | Kruskal Wallis p-value | Mann-Whitney (pairwise) p-value |
|---|
| IL-6 | Group A | 53 | 10.92 | 2.65 | <0.001*** | <0.001*** |
| Group B | 27 | 40.05 | 11.66 |
| Group C | 33 | 120.93 | 40.96 |
| Ferritin | Group A | 53 | 368.21 | 374.85 | 0.406 | 0.428 |
| Group B | 27 | 309.15 | 291.58 |
| Group C | 33 | 433.45 | 376.13 |
Group A patients were managed with Face mask/Nasal Prongs, Group B patients were managed with high flow nasal cannula/Bilevel Positive Airway Pressure (BIPAP), Group C patients required mechanical ventilation/high flow nasal cannula.
Discussion
In the present study, 113 RT-PCR confirmed COVID-19 infection cases, the patients had presented with mild symptoms to severe pneumonia. The patients with severe symptoms required ICU Admission. Around admitted to the ICU under mechanical ventilation died due progression of the COVID-19 infection to severe ARDS. In this cross-sectional analytical study, the levels of IL-6 were found to be significantly elevated in COVID-19 infected patients presented with severe symptoms, the mean levels of IL-6 was consistently increasing in mild to moderate and from moderate to severely infected COVID-19 patients. The findings in this study were in par with the retrospective study of dynamic changes in IL-6 levels in COVID-19 patients observed by Liu Z et al., on 738 COVID-19 Patients admitted in the hospital at Wuhan, China. The author observed increased levels of IL-6 that significantly correlated with disease severity and hospital mortality due to COVID-19 infection [1].
As far as authors have understood the pathogenesis of COVID-19 infection. The coronavirus binds with the Angiotensin Converting Enzyme 2 (ACE2) receptors on the surfactant producing Type II alveolar cells, ciliated and goblet cells in the airways [17-19]. The coronavirus effectively inhibits type 1 Interferon (IFN) signaling that will suppress the antiviral programs and subsequently activate innate and adaptive immune response that induces the release of Proinflammatory cytokines through NF kB pathway. The proinflammatory cytokines include IL-1, IL-6, and TNF α later increases the vascular permeability, causing influx of large amounts of fluid and blood cells into the alveoli causing ARDS [20,21].
In this study, any significant clinical correlation with ferritin levels among COVID-19 patients could not found. However, it was found that the serum ferritin levels were quiet high in severe cases as compared with mild to moderately symptomatic COVID-19 infection. The present study findings were in par with observations made by Zhou F et al., where Ferritin levels of >400 correlated significantly with severe infection and mortality due to COVID-19. The increased ferritin levels in patients with severe COVID-19 infection may indicate a secondary bacterial infection, resulting in increased synthesis and release of intracellular ferritin especially from the reticuloendothelial system during viral and bacterial infections [22].
Ferritin is a protein concerned with iron storage and homeostasis, serum ferritin is also an acute phase protein found to be elevated during infections, as a part of host defense mechanisms, hyperferritinaemia in infections is protective as it limits the production of free radicals and mediates immunomodulation [23]. Previous studies show hyperferritinemia in dengue virus infection to indicate a highly active disease with immune activation and coagulation disturbances [24,25].
Similarly, in COVID-19 the release of excessive of ferritin from cells is observed to be due to cytokine stimulus especially IL-6, as an account of viraemia there is significant activation of macrophages. This hyperferritinemia is seen in severe infections of COVID-19 and its levels correlates with high mortality [26,27]. This study could not find any significant correlation between serum IL-6 levels with Ferritin levels across disease severity taken together. However, there was a proportionate increase in the levels of IL-6 with regards to the severity in manifestations of COVID-19 infected patients in the present study. Hence, determining the IL-6 levels in COVID-19 patients can be a potentially useful in clinically correlating with the severity of the infection and timely management.
Limitation(s)
The influence of other co-morbid conditions and habits on the levels of IL-6 and Ferritin Levels that can affect the outcome of COVID-19 infection should also be considered in future studies with larger sample size.
Conclusion(s)
Serum IL-6 levels independently showed a good correlation with disease severity among COVID-19 patients, and serum ferritin levels was elevated only in severely symptomatic individuals with COVID-19 infections. Hence, Serum IL-6 could have a significant role in assessment of disease severity and prognosis among COVID-19 patients.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Feb 01, 2021
Manual Googling: Mar 26, 2021
iThenticate Software: Apr 12, 2021 (12%)
[1]. Liu Z, Li J, Chen D, Gao R, Zeng W, Chen S, Dynamic interleukin-6 level changes as a prognostic indicator in patients with COVID-19Frontiers in Pharmacology 2020 11:109310.3389/fphar.2020.0109332765283 [Google Scholar] [CrossRef] [PubMed]
[2]. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Clinical features of patients infected with 2019 novel coronavirus in Wuhan. ChinaThe Lancet 2020 395:497-506.10.1016/S0140-6736(20)30183-5 [Google Scholar] [CrossRef]
[3]. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Genomic characterization and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor bindingThe Lancet 2020 395(10224):565-74.10.1016/S0140-6736(20)30251-8 [Google Scholar] [CrossRef]
[4]. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Clinical and immunological features of severe and moderate coronavirus disease 2019The Journal of Clinical Investigation 2020 130(5):2620-29.10.1172/JCI13724432217835 [Google Scholar] [CrossRef] [PubMed]
[5]. Qin C, Zhou L, Hu Zhang S, Yang S, Tao Y, Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, ChinaClinical Infectious Diseases 2020 71(15):762-68.10.1093/cid/ciaa24832161940 [Google Scholar] [CrossRef] [PubMed]
[6]. Ji D, Zhang D, Chen Z, Xu Z, Zhao P, Zhang M, Clinical characteristics predicting progression of COVID-19The Lancet 2020 :01-18.10.2139/ssrn.3539674 [Google Scholar] [CrossRef]
[7]. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patientsE Bio Medicine 2020 55:01-10.10.1016/j.ebiom.2020.10276332361250 [Google Scholar] [CrossRef] [PubMed]
[8]. Liu T, Zhang J, Yang Y, Ma H, Li Z, Zhang J, The potential role of IL-6 in monitoring severe case of Coronavirus disease 2019EMBO Molecular Medicine 2020 12(7):01-24.10.15252/emmm.20201242132428990 [Google Scholar] [CrossRef] [PubMed]
[9]. Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARSEuropean Journal of Clinical Investigation 2009 39(7):618-25.10.1111/j.1365-2362.2009.02153.x19453650 [Google Scholar] [CrossRef] [PubMed]
[10]. Leth-Larsen R, Zhong F, Chow VT, Holmskov U, Lu J, The SARS Coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophagesImmunobiology 2007 212(3):201-11.10.1016/j.imbio.2006.12.00117412287 [Google Scholar] [CrossRef] [PubMed]
[11]. Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, Chemokine upregulation in SARS-coronavirus-infected, monocyte-derived human dendritic cellsBlood 2005 106(7):2366-74.10.1182/blood-2004-10-416615860669 [Google Scholar] [CrossRef] [PubMed]
[12]. Matsuyama R, Nishiura H, Kutsuna S, Hayakawa K, Ohmagari N, Clinical determinants of the severity of Middle East respiratory syndrome (MERS): A systematic review and meta-analysisBMC Public Health 2016 16(1):120310.1186/s12889-016-3881-427899100 [Google Scholar] [CrossRef] [PubMed]
[13]. Pastoraa JG, Weiganda M, Kima J, Wua X, Strayera J, Palmera AF, Hyperferritinemia in critically ill COVID-19 patients- Is ferritin the product of inflammation or a pathogenic mediator?Clinica Chimica Acta 2020 509:249-51.10.1016/j.cca.2020.06.03332579952 [Google Scholar] [CrossRef] [PubMed]
[14]. Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019Clinical Infectious Diseases 2020 71(8):1937-42.10.1093/cid/ciaa44932301997 [Google Scholar] [CrossRef] [PubMed]
[15]. Hozo B, Djulbegovic, Estimating the Mean and Variance from the Median, Range, and the Size of a SampleBMC Medical Research Methodology 2005 5:1310.1186/1471-2288-5-1315840177 [Google Scholar] [CrossRef] [PubMed]
[16]. Clinical management protocol: COVID-19 guidelines by Government of India, Ministry of Health and family welfare, Directorate General of Health services. Version 5.03.07.2020. Available at https://www.mohfw.gov.in [Google Scholar]
[17]. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, Goor HV, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesisJ Pathol 2004 203(2):631-37.10.1002/path.157015141377 [Google Scholar] [CrossRef] [PubMed]
[18]. Sims AC, Baric RS, Yount B, Burkett SE, Collins PL, Pickles RJ, Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: Role of ciliated cells in viral spread in the conducting airways of the lungsJ Virol 2005 79(24):15511-24.10.1128/JVI.79.24.15511-15524.200516306622 [Google Scholar] [CrossRef] [PubMed]
[19]. Sungnak WH, Becavin C, Berg M, SARS-CoV-2 entry genes are most highly expressed in nasal goblet and ciliated cells within human airwaysNature Medicine 2020 26:681-87.10.1038/s41591-020-0868-632327758 [Google Scholar] [CrossRef] [PubMed]
[20]. Knudsen L, Ochs M, The micromechanics of lung alveoli: Structure and function of surfactant and tissue componentsHistochem Cell Biol 2018 150(6):661-76.10.1007/s00418-018-1747-930390118 [Google Scholar] [CrossRef] [PubMed]
[21]. Leiva-Juarez MM, Kolls JK, Evans SE, Lung epithelial cells: Therapeutically inducible effectors of antimicrobial defenseMucosal Immunol 2018 11(1):21-34.10.1038/mi.2017.7128812547 [Google Scholar] [CrossRef] [PubMed]
[22]. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: A retrospective cohort studyLancet 2020 395(10229):1054-62.10.1016/S0140-6736(20)30566-3 [Google Scholar] [CrossRef]
[23]. Kernan KF, Carcillo JA, Hyperferritinemia and inflammationInternational Immunology 2017 29(9):401-09.10.1093/intimm/dxx03128541437 [Google Scholar] [CrossRef] [PubMed]
[24]. Van de Weg CAM, Huits RMHG, Pannuti CS, Brouns RM, van den Berg RWA, Hyperferritinaemia in dengue virus infected patients is associated with immune activation and coagulation disturbancesPLoS Negl Trop Dis 2014 8(10):e321410.1371/journal.pntd.000321425299654 [Google Scholar] [CrossRef] [PubMed]
[25]. Krishnamurti C, Peat RA, Cutting MA, Rothwell SW, Platelet adhesion to dengue-2 virus-infected endothelial cellsThe American Journal of Tropical Medicine and Hygiene 2002 66(4):435-41.10.4269/ajtmh.2002.66.43512164302 [Google Scholar] [CrossRef] [PubMed]
[26]. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson J, COVID-19: Consider cytokine storm syndromes and immunosuppressionLancet 2020 395(10229):1033-34.10.1016/S0140-6736(20)30628-0 [Google Scholar] [CrossRef]
[27]. Vargas-Vargas M, Cortés-Rojo C, Ferritin levels and COVID-19Rev Panam Salud Publica 2020 44:e7210.26633/RPSP.2020.7232547616 [Google Scholar] [CrossRef] [PubMed]