Topical Ripasudil as First Line Treatment for Ocular Hypertension in Uveitis Cases: A Prospective Study
Harvinder Nagpal1, Mandeep Kaur2
1 Associate Professor, Department of Ophthalmology, Government Medical College, Patiala, Punjab, India.
2 Senior Resident, Department of Ophthalmology, Government Medical College, Patiala, Punjab, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Harvinder Nagpal, 133, Kanika Garden, Rajpura, Patiala, Punjab, India.
E-mail: drnagpal.2009@gmail.com
Introduction
Uveitis can directly or indirectly lead to ocular hypertension which can lead to glaucoma. Ripasudil has a different mechanism of action than other anti-glaucoma medications available commercially and in contrary to these drugs, ripasudil also has anti-inflammatory properties providing an upper edge over other intraocular pressure lowering drugs. In addition to primary glaucoma, these new Rho kinase associated inhibitors can provide satisfactory results in glaucoma with secondary pathologies.
Aim
To study the role of ripasudil as first line treatment for ocular hypertension in uveitis cases.
Materials and Methods
A prospective randomised study comprising 40 patients of Ocular Hypertension (OHT) associated with uveitis was conducted in the Outpatient Department (OPD) of Ophthalmology at a tertiary care hospital in North India from October 2020 to January 2021. The diagnosis of uveitis was made clinically with detailed medical history and slit lamp biomicroscopic examination. All the diagnosed patients were started with topical ripasudil hydrochloride hydrate 0.4% eye drops twice daily along with anti-inflammatory medications. Intraocular Presure (IOP) was recorded after 4 weeks and 12 weeks at 8 am, 10 am and 4 pm. Effectiveness of the drugs was calculated in terms of mmHg fall in mean intraocular pressure using t-test and p-values.
Results
Out of 40 patients with uveitis associated OHT, 20 cases were inflammation related (mean age was 56.2±16.3 years, 13 males, 7 females) and 20 cases were categorised as corticosteroid induced (mean age was 58.9±15.69 years, 14 males, 6 females). At 12 weeks there was 5.67±0.59 mmHg fall in IOP (22.70%) in inflammation related OHT and there was 6.37±0.07 mmHg fall in IOP (25.34%) in corticosteroid related OHT. There was statistically significant fall in IOP (p-value=0.001).
Conclusion
This study demonstrated that topical ripasudil hydrochloride hydrate 0.4% eye drops is effective in lowering the IOP, also there were no side effects, so it is safe and well-tolerated. So, ripasudil can provide a safe and effective alternative for lowering of IOP among uveitis related OHT.
Corticosteroids, Intraocular pressure, Inflammation
Introduction
Inflammation of iris, ciliary body and/or choroid is referred to as uveitis which can result from variable causes, both infective and non-infective [1,2]. Uveitis can directly or indirectly lead to raised Intraocular Pressure (IOP) leading to Ocular Hypertension (OHT) and persistent rise of IOP can lead to optic neuropathy and loss of visual field that is termed as uveitic glaucoma [3]. This rise of IOP can be both acute and chronic in nature, either due to obstruction of trabecular meshwork by inflammatory processes or scarring due to recurrent inflammatory insults to trabecular outflow. Moreover, corticosteroids used to treat uveitis can themselves contribute to imbalance of IOP control [4]. Medical management of IOP rise is too crucial along with managing uveitis to prevent progression into glaucoma, thus joint treatment should be focused to minimise ocular morbidity.
Recently newer anti-glaucoma drug group was approved by Food and Drug Administration (FDA) comprising Rho-associated inhibitors (netrasudil and ripasudil) and they increase trabecular outflow, possibly by expanding the intertrabecular spaces and relaxing the trabecular meshwork [5,6]. Commercially available ripasudil hydrochloride hydrate has also been postulated to have anti-inflammatory properties [7], hence can provide an edge over other IOP lowering drugs in uveitis cases. In this study, role of ripasudil as first line drug to lower IOP in uveitis cases was studied.
Materials and Methods
A prospective randomised study comprising 40 patients of ocular hypertension associated with uveitis with age between 20-60 years was conducted in the Outpatient Department (OPD) of Ophthalmology at a tertiary care hospital in North India. This study was done after approval from Institutional Review Board of Government Medical College, Patiala (REC/GMC/1231-20).
All the patients satisfying inclusion criteria were included in the study after obtaining an informed consent and those with exclusion criteria were excluded from the study [Table/Fig-1]. The diagnosis of uveitis was made clinically with detailed medical history and slit lamp biomicroscopic examination. All the cases were classified in accordance to latest definitions by International Uveitis Study Group (IUSG) [8,9].
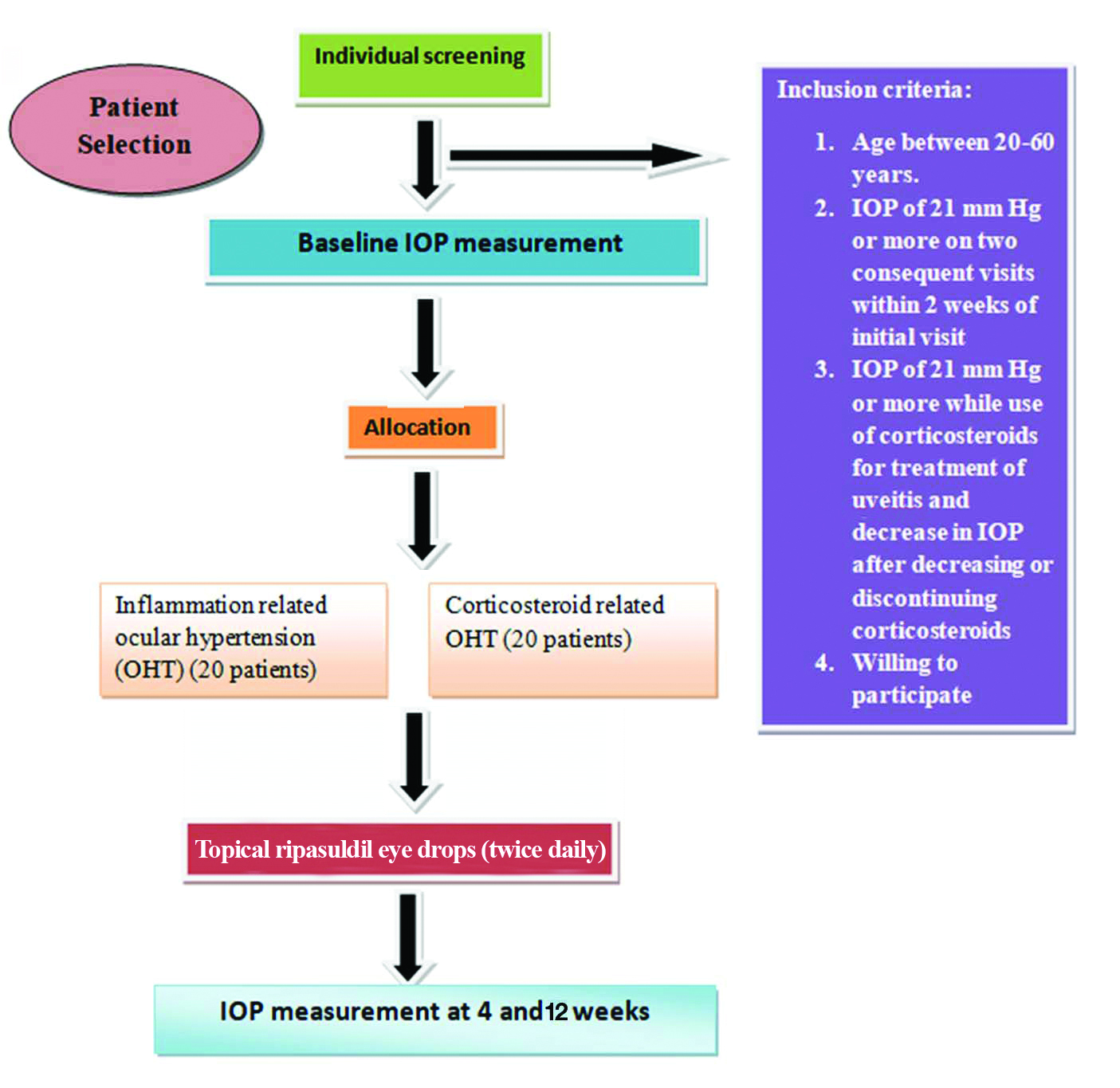
Inclusion criteria: Inflammatory OHT was considered in cases with IOP of 21 mmHg or more on two consequent visits within two weeks of initial visit and those with corticosteroid induced OHT was considered with IOP of 21 mmHg or more while use of corticosteroids for treatment of uveitis and decrease in IOP after decreasing or discontinuing corticosteroids were included in the study. This differentiation was difficult in some cases so decision was made according to the clinical expertisae.
Exclusion criteria: Patients with corneal pathologies, any other primary or secondary glaucoma, pseudoexfoliation or pigmentary glaucoma, hypersensitivity or contraindication to the study medication, history of ocular trauma or surgery were excluded from the study.
Study Procedure
One eye (the affected eye) fulfilling the inclusion criteria was considered as the study eye. If both eyes had glaucoma then both eyes were treated but only one eye was randomly selected to be the study eye. A detailed ocular and medical history was obtained. Careful general physical examination was done to rule out any contraindications to the drugs. Ocular examination included recording of Best Corrected Visual Acuity (BCVA) with a snellen chart, examination of lids, adnexa, and lacrimal apparatus using diffuse light, biomicroscopy of anterior segment using slit lamp to note aqueous flare and aqueous cells. Further direct ophthalmoscopy, goldmann applanation tonometry and slit lamp biomicroscopy with +78D or +90D lens was also done to assess cup disc ratio (C:D ratio), neuroretinal rim health and retinal nerve fibre layer on all visits. Baseline IOP was recorded at OPD of Department of Ophthalmology before starting the treatment. All the patients were started on topical ripasudil hydrochloride hydrate 0.4% eye drops twice daily along with anti-inflammatory medications. IOP was recorded after 4 weeks and 12 weeks at 8 am, 10 am and 4 pm. Effectiveness of the drugs was calculated in terms of mmHg fall in mean intraocular pressure.
Statistical Analysis
All the observations made were compiled on a proforma, subjected to statistical analysis using t-test and p-values were calculated. A p-value of less than 0.05 was considered stastically significant, p-value less than 0.01 was highly significant.
Results
Out of 40 patients with uveitis associated OHT, 20 cases were inflammation related (mean age was 56.2±16.3 years,13 males, 7 females) and 20 cases were categorised as corticosteroid induced (mean age was 58.9±15.69 years, 14 males, 6 females). Age and gender when compared among both set of patients, results were found to be non significant. There were no side effects after 12 weeks. [Table/Fig-2] shows the mean diurnal reduction in IOP in 20 patients with inflammation related OHT, at 4 weeks there was 4.67±0.37 mmHg fall in IOP (18.75%) and at 12 weeks there was 5.67±0.59 mmHg fall in IOP (22.70%).
Mean Diurnal IOP changes in inflammation related OHT on subsequent visits.
| Visits | Mean±SD | Difference | Percentage Reduction | t-test | p-value |
|---|
| Baseline | 24.97±2.18 | --- | --- | --- | --- |
| 4 weeks | 20.30±1.81 | 4.67±0.37 | 18.75 | 23.568 | 0.001 |
| 12 weeks | 19.30±1.59 | 5.67±0.59 | 22.70 | 23.459 | 0.001 |
p-value <0.05 considered significant; p-value <0.01 considered highly significant
[Table/Fig-3] shows the mean diurnal reduction in IOP in 20 patients with corticosteroid related OHT, at 4 weeks there was 5.22±0.04 mmHg fall in IOP (20.77%) and at 12 weeks there was 6.37±0.07 mmHg fall in IOP (25.34%). None of the patients missed any follow-up examinations.
Mean Diurnal IOP changes in corticosteroid related OHT on subsequent visits.
| Visits | Mean±SD | Difference | Percentage reduction | t-test | p-value |
|---|
| Baseline | 25.13±1.58 | --- | --- | --- | --- |
| 4 weeks | 19.91±1.62 | 5.22±0.04 | 20.77 | 27.887 | 0.001 |
| 12 weeks | 18.76±1.65 | 6.37±0.07 | 25.34 | 29.169 | 0.001 |
p-value <0.05 considered significant; p-value <0.01 considered highly significant
[Table/Fig-4] shows the mean diurnal reduction in IOP in both set of patients that is inflammation related and corticosteroid related OHT, results were statistically insignificant.
Mean Diurnal IOP Changes at various visits among both set of patients.
| Visits | Inflammation related OHT | Corticosteroid related OHT | t-test | p-value |
|---|
| Mean IOP | Reduction of mean diurnal IOP from baseline | Mean IOP | Reduction of mean diurnal IOP from baseline |
|---|
| Difference | Percentage | Difference | Percentage |
|---|
| Baseline | 24.97±2.18 | --- | --- | 25.13±1.58 | --- | --- | 0.339 | 0.736 |
| 4 weeks | 20.30±1.81 | 4.67±0.37 | 18.75 | 19.91±1.62 | 5.22±0.04 | 20.77 | 0.874 | 0.386 |
| 12 weeks | 19.30±1.59 | 5.67±0.59 | 22.70 | 18.76±1.65 | 6.37±0.07 | 25.34 | 1.270 | 0.209 |
p-value <0.05 considered significant; p-value <0.01 considered highly significant
Discussion
Ripasudil hydrochloride hydrate 0.4% eye drops belongs to group of newly developed and approved anti-glaucoma drug class named Rho associated kinase inhibitors. As uveitis is an inflammatory ocular condition associated with acute or chronic rise of intraocular pressure and this persistent rise of IOP can lead to glaucoma . Along with controlling and effectively decreasing IOP, ripasudil does not worsen up the uveitis [10]. In the present study, cases are classified as the cases of OHT associated with uveitis as consequent to inflammation or due to corticosteroid response.
Tanihara H et al., [11] studied the role of ripasudil for lowering IOP in cases of primary open angle glaucoma and documented a total 3.7 mmHg fall of IOP. Tsukahara S et al., [12] used ripasudil as either add on therapy or replacement drug in glaucoma patients, they documented 3 mmHg fall in IOP with p-value <0.05. Yasuda M et al., [13] studied role of ripasudil in OHT associated with uveitis and concluded 8.5 mmHg fall in IOP in inflammation related cases and 8.1 mmHg fall in IOP in corticosteroid related cases [Table/Fig-5]. While in present study, at 12 weeks there was 5.67±0.59 mmHg fall in IOP (22.70%) in inflammation related ocular hypertension (OHT) and there was 6.37±0.07 mmHg fall in IOP (25.34%) in corticosteroid related OHT. There was statistically significant fall in IOP (p-value=0.001). Corticosteroids damage the trabecular meshwork at cellular level leading to resistance for aqueous outflow, thus leading to raised IOP [14]. In present study, there was a significant fall of IOP (6.37 mmHg) in corticosteroid related OHT and thus give a positive inference that ripasudil seem appropriate and safe drug for IOP control in uveitis cases.
Comparison of mmHg fall in IOP from previous studies.
| Study | mmHg fall in IOP |
|---|
| Tanihara H et al., [11] | 3.7 |
| Tsukahara S et al., [12] | Additional 3 mmHg fall in IOP (as an adjunct to previous treatment) |
| Yasuda M et al., [13] | 8.5 (inflammation related ocular hypertension) 8.1 (corticosteroid related OHT) |
| Present study | 5.67 (inflammation related ocular hypertension) 6.37 (corticosteroid related OHT) |
Limitation(s)
Limitation of the study was small sample size, future study can be done with large sample size.
Conclusion(s)
This study demonstrated that topical ripasudil hydrochloride hydrate 0.4% eye drops is effective in lowering the IOP, also there were no side effects, so it is safe and well-tolerated. The results conclude that ripasudil can provide a safe and effective alternative for IOP lowering among uveitis related OHT cases.
p-value <0.05 considered significant; p-value <0.01 considered highly significant
p-value <0.05 considered significant; p-value <0.01 considered highly significant
p-value <0.05 considered significant; p-value <0.01 considered highly significant
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Feb 06, 2021
Manual Googling: Apr 18, 2021
iThenticate Software: Apr 03, 2021 (8%)
[1]. Snell RS, Lemp MA, Clinical Anatomy of the Eye 1998 2nd edBlackwell Publishing:140-156.10.1002/9781118690987 [Google Scholar] [CrossRef]
[2]. Rao NA, Forster DJ, The Uvea, Uveitis, and Intraocular Neoplasms 1992 New YorkGower Medical Publications:01-17. [Google Scholar]
[3]. Bodh SA, Kumar V, Raina UK, Ghosh B, Thakar M, Inflammatory glaucomaOman J Ophthalmol 2011 4(1):03-09.10.4103/0974-620X.7765521713239 [Google Scholar] [CrossRef] [PubMed]
[4]. Armaly MF, Effect of corticosteroids on intraocular pressure and fluid dynamics. I. The effect of dexamethasone in the normal eyesArch Ophthalmol 1963 70:48210.1001/archopht.1963.0096005048401014078870 [Google Scholar] [CrossRef] [PubMed]
[5]. Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facilityInvest Ophthalmol Vis Sci 2001 42:137-44. [Google Scholar]
[6]. Rao PV, Deng PF, Kumar J, Epstein DL, Modulation of aqueous humor outflow facility by the rho kinase-specific inhibitor Y-27632Invest Ophthalmol Vis Sci 2001 42:1029-37. [Google Scholar]
[7]. Uchida T, Honjo M, Yamagishi R, Aihara M, The Anti-Inflammatory Effect of Ripasudil (K-115), a Rho Kinase (ROCK) Inhibitor, on Endotoxin-Induced Uveitis in RatsInvest Ophthalmol Vis Sci 2017 58:5584-93.10.1167/iovs.17-2267929084331 [Google Scholar] [CrossRef] [PubMed]
[8]. Deschenes J, Murray PI, Rao NA, Nussenblatt RB, International Uveitis Study Group (IUSG) clinical classification of uveitisOcul Immunol Inflamm 2008 16:01-02.10.1080/0927394080189982218379933 [Google Scholar] [CrossRef] [PubMed]
[9]. Jabs D, Nussenblatt R, Rosenbaum J, Standardization of uveitis nomenclature for reporting clinical dataAm J Ophthalmol 2005 140:509-16.10.1016/j.ajo.2005.03.05716196117 [Google Scholar] [CrossRef] [PubMed]
[10]. Yamada H, Yoneda M, Inaguma S, Gosho M, Murasawa Y, Isogai Z, A Rho-associated kinase inhibitor protects permeability in a cell culture model of ocular disease, and reduces aqueous flare in anterior uveitisJ Ocul Pharmacol Ther 2017 33:176-185.10.1089/jop.2016.008528157424 [Google Scholar] [CrossRef] [PubMed]
[11]. Tanihara H, Inoue T, Yamamoto T, Kuwahara Y, Abe H, Fukushima A, One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertensionActa Ophthalmol 2016 94(1):26-34.10.1111/aos.1282926338317 [Google Scholar] [CrossRef] [PubMed]
[12]. Tsukahara S, Enomoto N, Ishida K, Anraku A, Tomita G, One-Year Efficacy and Safety Assessment of Ripasudil, a Rho Kinase Inhibitor, in an Addition to or Replacing Existing Treatment Regimens: A Retrospective StudyJournal of Ocular Pharmacology and Therapeutics 2020 36:512-21.10.1089/jop.2019.008932412867 [Google Scholar] [CrossRef] [PubMed]
[13]. Yasuda M, Takayama K, Kanda T, Taguchi M, Someya H, Takeuchi M, Comparison of intraocular pressure-lowering effects of ripasudil hydrochloride hydrate for inflammatory and corticosteroid-induced ocular hypertensionPLoS ONE 2017 12(10):e018530510.1371/journal.pone.018530528968412 [Google Scholar] [CrossRef] [PubMed]
[14]. Underwood JL, Murphy CG, Chen J, Franse-Carman L, Wood I, Epstein DL, Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctionsAm J Physiol 1999 277:330-42.10.1152/ajpcell.1999.277.2.C33010444410 [Google Scholar] [CrossRef] [PubMed]