Endobronchial Hamartoma with Postobstructive Pneumonia- A Case of Successful Treatment with Endobronchial Interventions
Jolsana Augustine1, Rajesh Venkitakrishnan2, VR Pattabhiraman3, Arjun Srinivasan4
1 Consultant, Department of Pulmonology, Rajagiri Hospital, Kochi, Kerala, India.
2 Consultant, Department of Pulmonology, Rajagiri Hospital, Kochi, Kerala, India.
3 Consultant, Department of Interventional Pulmonology, Royal Care Hospital, Coimbatore, Tamil Nadu, India.
4 Consultant, Department of Interventional Pulmonology, Royal Care Hospital, Coimbatore, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Jolsana Augustine, Consultant Pulmonologist, Rajagiri Hospital, Aluva, Kochi-683112, Kerala, India.
E-mail: j_augustine08@ymail.com
Airway hamartomas are of mesenchymal tissue origin and have predominance of adipose tissue. Hamartoma refers to a tumour-like structure within an organ composed of an abnormal arrangement of tissue components normally found in that organ. The pulmonary parenchyma and airways are commonly described sites for this lesion, although endobronchial location is far less common than its parenchymal counterpart. When present within a major airway, the usual symptoms of an airway lesion like cough, wheezing, stridor, haemoptysis and postobstructive consolidation ensue. Here, authors describe the case of a 60-year-old man who presented with cough from past 9 months. On evaluation he was found to have an endobronchial lesion in the right main bronchus. The histopathology revealed features of endobronchial hamartoma which was then subjected to endobronchial intervention with electrocautery and cryoablation achieving complete removal and restoration of luminal patency. This case underscores the ability of endobronchial treatment modalities to successfully treat benign airway lesions like hamartoma when undertaken in experienced centres.
Bronchial hamartoma, Bronchial tumour, Cryoablation, Endobronchial treatment
Case Report
A 60-year-old man with no co-morbidities presented to the Pulmonary Medicine Outpatient Department of our hospital with cough from past 9 months. The cough was not associated with any expectoration. He experienced breathing difficulty and wheezing sound while breathing. There was no fever, weight loss, shortness of breath or voice change. There was worsening of cough with fever and right sided pleuritic chest pain for 3 days. He never experienced similar symptoms in the past. Family history was not relevant except for mild allergic symptoms among siblings. Physical evaluation revealed a no respiratory distress. Chest examination was notable for fine crackles in the right lower chest and a monophonic wheeze over the same area.
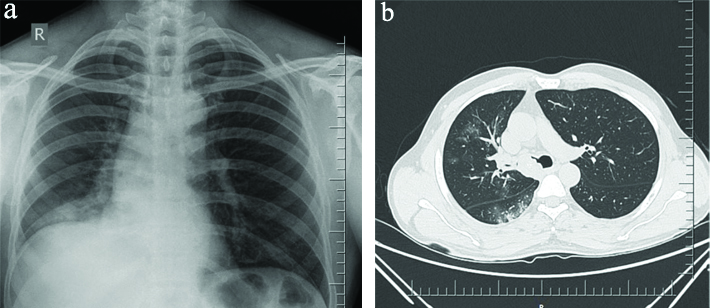
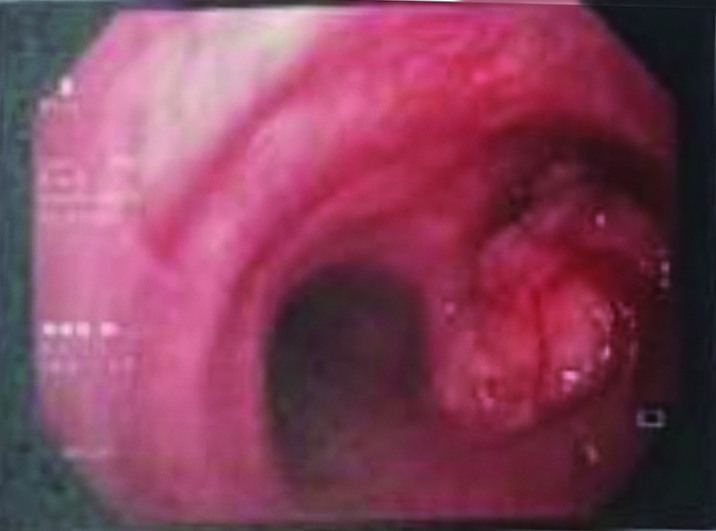
Routine blood investigations (Complete blood count, renal and liver function parameters) were within normal limits. His chest radiograph postero anterior view is depicted in [Table/Fig-1a], which shows right lower zone alveolar infiltrates with elevated right hemi diaphragm suggesting lower right hemithorax volume loss. CT thorax showed a well-defined soft tissue density mass lesion with irregular margins within the lumen of proximal right main bronchus and secretions within right lower lobe bronchus causing near complete occlusion of lumen, resulting in areas of collapse-consolidation with surrounding ground glass opacities in the basal segments of right lower lobe [Table/Fig-1b]; the lesion depicted focal calcification. Radiologist suggested a contrast study which the relatives opted out. The differential diagnosis were bronchogenic carcinoma and intrabronchial carcinoids. He was initiated on broad spectrum antibiotics for his right lower lobe consolidation and a fibre optic bronchoscopy was undertaken. Bronchoscopy depicted an approximately 1 cm exophytic, well circumscribed lesion in the right main bronchus [Table/Fig-2]. Authors could not manipulate beyond right main bronchus as thin 3.4 mm scope was not available at that time. Thorough saline lavage was performed and purulent secretions were retrieved and sent for microbiological studies like bacterial, mycobacterial and fungal culture. Multiple biopsies were attempted form this lesion. The lesion seemed hard to touch on attempting a biopsy. No active bleed was noted. Histopathology evaluation was suggestive of a benign lesion-endobronchial hamartoma.
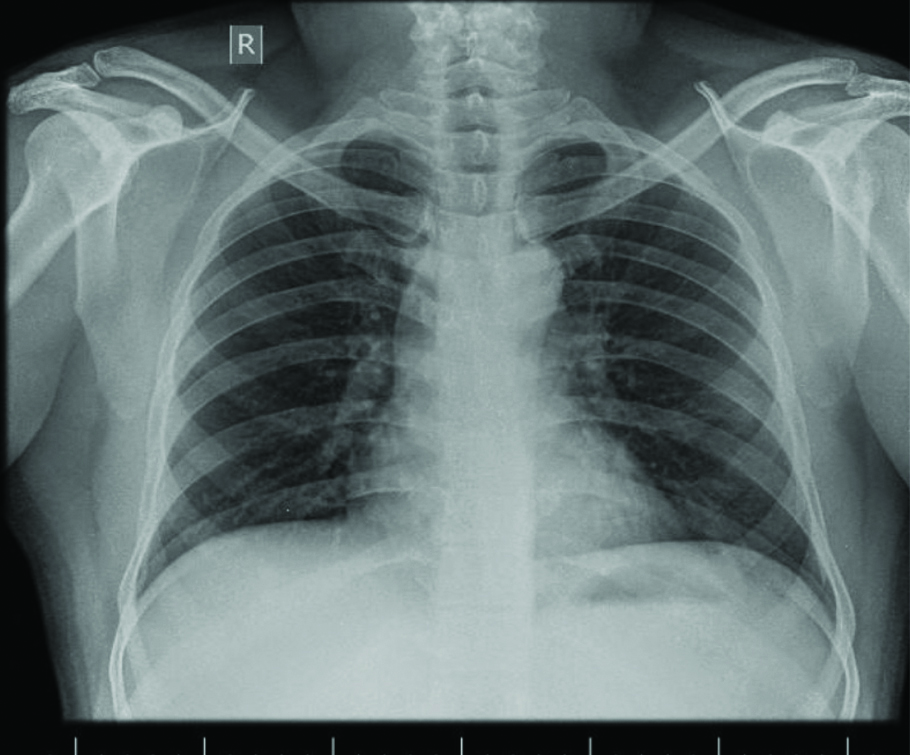
Since surgery was not acceptable to patient, he opted for endobronchial interventional modalities after proper counselling and consent. Rigid bronchoscopy was performed under general anaesthesia using 11 sized tracheobronchoscope (Olympus BF TQ 170). The tumour was mechanically debulked and snared when the base of the lesion could be visualised. The debulked pieces were cleared using 2.4 mm cryoprobe (ERBECRYO 2). Total restoration of luminal patency was achieved. Postprocedure small mucosal dehiscence was noted along the posterior wall of right main bronchus corresponding to the base of the lesion. No active bleeding was noted and he was extubated. Chest radiograph postprocedure [Table/Fig-3] showed full clearance of right lower lobe collapse consolidation. He was discharged home 48 hours after the interventional procedure on oral antibiotics, bronchodilators and antitussives. A check bronchoscopy performed a week after the procedure showed essentially an unobstructed bronchial tree. He was kept on regular follow-up for one-year postprocedure the last follow-up being 10 months back. He is not on any regular medications for the same.
a) Chest radiograph postero anterior view; b) Areas of collapse-consolidation with surrounding ground glass opacities in the basel segments of right lower lobe.
Bronchoscopy depicted an approximately 1 cm exophytic, well circumscribed lession in the right main brochus.
Chest radiograph post procedure.
Discussion
Hamartomas are benign tumour-like malformation made up of varying proportions of cartilage, fat, fibrous tissue and entrapped respiratory epithelium. Only 10% of pulmonary hamartomas occurs endobronchially, while the rest are peripheral. The term hamartoma has its origin from the Greek word hamartia, meaning “fault, defect,” and oma, denoting a tumour or neoplasm [1]. Pulmonary hamartomas are rare lesions whose population incidence is unknown in the Indian subcontinent. Hamartomas are the second most common benign neoplasm of the pulmonary system and in one large series they were found to be 8% in central airways [2,3]. The vast majority of pulmonary hamartomas are intraparenchymal and peripheral [4]. The peak incidence of occurrence of hamartoma was in the seventh decade of life [5]. Hamartomas, as opposed to other benign endobronchial tumours like chondromas or lipomas, are characterised by the presence of atleast two mesenchymal elements like cartilage or fat. Genetic rearrangement of chromosome band 12q15 is frequently found in pulmonary hamartoma [6].
Endobronchial hamartomas, when compared to their peripheral parenchymal counterparts are usually symptomatic. Persistent cough is a common presenting symptom. It can be associated with obstruction of the bronchus resulting in postobstructive pneumonia and lobar atelectasis [7,8]. Haemoptysis may occur if there is vascular invasion or perforation by tumour [9].
Chest radiographs findings are non specific in contrast to parenchymal lesions, where a nodular lesion with the characteristic popcorn calcification can be reasonably specific as to be diagnostic. Chest CT shows collections of fat alternating with foci of calcification [2]. It typically contains more fat tissue than parenchymal elements. Endobronchial hamartomas grossly appear as broad-based, polypoid nodules within the lumen of a large bronchus. They are white or grey in colour with a firm consistency. Histologically, pulmonary hamartomas predominantly contain cartilage as well as variable amounts of fibromyxoid connective tissue, fat, bone, and smooth muscle in decreasing order of frequency [10].
The management of endobronchial hamartoma must be individualised according to the characteristics of each patient and the location of the tumour. Traditionally, surgical resection was considered the gold standard treatment for endobronchial hamartomas [11]. However, there are increasing publications supporting bronchoscopic treatment modalities such as Nd:YAG laser and electrocautery having comparable results with surgical resection and very few reported complications [12-14]. In a five year retrospective study of data of patients with endobronchial tumours demonstrated 77% success rate with interventional techniques via bronchoscopy [15].
A large retrospective analysis revealed 17 out of 135 patients having endobronchial hamartoma. Fifteen patients were treated with rigid or flexible bronchoscopic resection and only 1 had to undergo surgical resection. There were no complications identified immediate or late [16]. Bronchoscopic electrocautery resection is used in patients where malignancy is not suspected and lesion is small. Surgical intervention is warranted when lesion could not be differentiated from malignancy and in cases with recurrence after initial bronchoscopic intervention [13].
Successful treatment of endobronchial hamartoma using a combination of cryotherapy and electrocautery has also been previously reported [17]. Cryotherapy is the application of extreme cold energy to diseased tissue, in which cells are destroyed by the formation of intracellular ice crystals. Cryotherapy can be delivered through rigid or flexible bronchoscopy. Endoscopic techniques are safe, cheaper and less invasive in experienced hands. Therefore, it should be considered as the primary treatment approach in selected cases. In cases of chronic cough usual causes need to be ruled out first. If unrewarding, we should look endobronchially for any cause of chronic cough.
Conclusion(s)
The case report summarises about an elderly man who presented with undiagnosed chronic cough. Physical evaluation with thoracic imaging revealed an endobronchial lesion in the right main bronchus extending into the carina with right lower lobe collapse consolidation. The lesion proved to be an endobronchial hamartoma on pathological examination, which was successfully removed with a combination of endobronchial cryotherapy and mechanical debulking, thereby restoring total airway patency. The case highlights the importance of looking for the presence of endobronchial lesions in cases of chronic nonresolving cough.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 18, 2020
Manual Googling: Aug 26, 2020
iThenticate Software: Jan 05, 2021 (18%)
[1]. Gaerte SC, Meyer CA, Winer-Muram HT, Tarver RD, Conces DJ, Fat-containing lesions of the chestRadiographics 2002 22(suppl_1):S61-78.10.1148/radiographics.22.suppl_1.g02oc08s6112376601 [Google Scholar] [CrossRef] [PubMed]
[2]. Suut S, Al-Ani Z, Allen C, Rajiah P, Durr-e-Sabih AL-Harbi A, Pictorial essay of radiological features of benign intrathoracic massesAnn Thorac Med 2015 10(4):231-42. [Google Scholar]
[3]. Thomas JW, Staerkel GA, Whitman GJ, Pulmonary hamartomaAJR Am J Roentgenol 1999 172(6):164310.2214/ajr.172.6.1035030810350308 [Google Scholar] [CrossRef] [PubMed]
[4]. Gjevre JA, Myers JL, Prakash UB, Pulmonary hamartomasMayo Clin Proc 1996 71(1):14-20.10.4065/71.1.148538225 [Google Scholar] [CrossRef] [PubMed]
[5]. Sibala JL, Endobronchial hamartomasChest 1972 62(5):631-34.10.1378/chest.62.5.6315082044 [Google Scholar] [CrossRef] [PubMed]
[6]. Fletcher JA, Longtine J, Wallace K, Mentzer SJ, Sugarbaker DJ, Cytogenetic and histologic findings in 17 pulmonary chondroid hamartomas: Evidence for a pathogenetic relationship with lipomas and leiomyomasGenes Chromosomes Cancer 1995 12(3):220-23.10.1002/gcc.28701203107536462 [Google Scholar] [CrossRef] [PubMed]
[7]. Zehani-Kassar A, Ayadi-Kaddour A, Marghli A, Ridene I, Kilani T, El Mezni F, Clinical characteristics of resected bronchial hamartoma. Study of seven casesRev Mal Respir 2011 28(5):647-53.10.1016/j.rmr.2010.12.00621645835 [Google Scholar] [CrossRef] [PubMed]
[8]. Oguma T, Takiguchi H, Niimi K, Tomomatsu H, Tomomatsu K, Hayama N, Endobronchial Hamartoma as a Cause of PneumoniaAm J Case Rep 2014 15:388-92.10.12659/AJCR.89086925208559 [Google Scholar] [CrossRef] [PubMed]
[9]. Sharkey RA, Mulloy EMT, O’neill SJ, Endobronchial hamartoma presenting as massive haemoptysisEur Respir J 1996 9(10):2179-80.10.1183/09031936.96.091021798902487 [Google Scholar] [CrossRef] [PubMed]
[10]. Ahmed S, Arshad A, Mador MJ, Endobronchial hamartoma; a rare structural cause of chronic coughRespir Med Case Rep 2017 22:224-27.10.1016/j.rmcr.2017.08.01928913162 [Google Scholar] [CrossRef] [PubMed]
[11]. Borro JM, Moya J, Botella JA, Padilla JD, Canto A, Paris F, Endobronchial Hamartoma: Report of 7 CasesScand J Thorac Cardiovasc Surg 1989 23(3):285-87.[Internet]. [cited 2019 Aug 27]. Available from: https://www.tandfonline.com/doi/abs/10.3109/1401743890910601110.3109/140174389091060112617250 [Google Scholar] [CrossRef] [PubMed]
[12]. Altin S, Dalar L, Karasulu L, Cetinkaya E, Timur S, Solmazer N, Resection of giant endobronchial hamartoma by electrocautery and cryotherapy via flexible bronchoscopyTuberk Toraks 2007 55(4):390-94. [Google Scholar]
[13]. Guo W, Zhao YP, Jiang YG, Wang RW, Ma Z, Surgical treatment and outcome of pulmonary hamartoma: A retrospective study of 20-year experienceJ Exp Clin Cancer Res CR 2008 27:810.1186/1756-9966-27-818577258 [Google Scholar] [CrossRef] [PubMed]
[14]. Liu C, Wang JJ, Zhu YH, Chen C, Successful use of snare electrocautery via flexible fiberoptic bronchoscopy for removal of an endobronchial hamartoma causing chronic lung atelectasis and mimicking malignancyTher Adv Respir Dis 2017 11(12):435-38.10.1177/175346581773674529202683 [Google Scholar] [CrossRef] [PubMed]
[15]. Özgül MA, Çetinkaya E, Gül ş, Dinçer HE, Acat M, Boyacı H, Interventi per il trattamento di amartoma e lipoma endobronchialiEur J Oncol 2016 21(2):108-12. [Google Scholar]
[16]. Kim SA, Um SW, Song JU, Jeon K, Koh WJ, Suh GY, Bronchoscopic features and bronchoscopic intervention for endobronchial hamartomaRespirology 2010 15(1):150-54.10.1111/j.1440-1843.2009.01662.x19947992 [Google Scholar] [CrossRef] [PubMed]
[17]. Ucar N, Akpinar S, Aktas Z, Sipit T, Ozaydin E, Resection of endobronchial hamartoma causing recurrent hemoptysis by electrocautery and cryotherapyHippokratia 2014 18(4):355-56. [Google Scholar]