Introduction
Immunisation services are affected by Coronavirus Disease-2019 (COVID-19) pandemic due to lockdown and fear of COVID among public along with problems of workforce management and vaccine supply. All these factors have led to decrease in vaccination coverage. It may further lead to increase in risk of vaccine preventable diseases.
Aim
To assess the overall trend and evaluate the vaccination coverage during COVID-19 pandemic in a tertiary care hospital in Gwalior district.
Materials and Methods
This cross-sectional study was conducted on record based secondary data from the immunisation Out Patient Department (OPD) of the tertiary care hospital Gwalior, Madhya Pradesh, India, for the period of 1st February 2020 to 31st August 2020 was utilised in current study. Data were entered into Microsoft Excel version-2007 and analysed. Frequency, percentage, mean, and standard deviation were calculated as a descriptive measures and graphical presentation to show time trends. The p-value was calculated at 5% level of significance.
Results
In the study, 817 children were included, with mean age of 7.46±13.59 months. Total 61.1% (499) children were male and 38.9% (318) were female. During the study period, all the children were vaccinated for Bacille Calmette-Guerin (BCG) (10.6%) Pentavalent-1 series (36.4%), Pentavalent-2 series (17.4%), Pentavalent-3 series (12.2%), Measles-Rubella (MR-1) (6.5%), Booster-I (11.8%) and Booster-II (5.1%). Delayed vaccination was seen in 51 (6.20%) children. The delay was observed for Pentavalent-2 series (43.1%), pentavalent-3 series (51.0%) and MR-1 (5.9%) and the difference is significant at p-value <0.05.
Conclusion
Immunisation services were severely interrupted and completely suspended in April 2020. Certain amount of delay in various doses was also observed. Maintaining routine immunisation is essential in preventing an outbreak of vaccine preventable diseases.
Introduction
COVID-19 was declared a pandemic by World Health Organisation (WHO) on 11th March 2020 [1], since then it has affected large number of people across the globe. Currently, there are approximately 84 million [2] cases throughout the globe but still we do not have any specific treatment for it. The world has been affected in many different ways, especially the healthcare sector where even the developed countries claiming to have a better health infrastructure and health budget have shown lacunae in their system. Childhood Immunisation is one of the routine health care services that is greatly influenced, interrupted, delayed, re-organised, or completely suspended due to COVID-19 pandemic. Government has implemented various strategies to control the rate and spread of COVID-19 infection among its population. Strategies like home quarantine, total lockdown has resulted in decreased accessibility to routine Immunisation services [3].
Immunisation is one of the most cost-effective and successful public health interventions to-date. Every $1 invested in child Immunisation is projected to yield a return of $16 resulting from illnesses averted and upto $44 if the value of living longer and healthier lives were measured [4]. Since Immunisation system relies on functioning health facilities and stable communities to be effective so numerous high as well as low and middle-income countries are now experiencing a rapid decline in childhood immunisation coverage rates, leaving children at higher risk of vaccine preventable diseases and its complications [3].
A number of countries postponed Immunisation campaigns in the first five months of the pandemic, including: measles or measles-containing vaccines in 27 countries, inactivated polio vaccine in seven countries, bivalent, or monovalent oral poliovirus vaccine in 39 countries, meningococcal conjugated A vaccine in two countries, yellow fever vaccine in four countries, typhoid vaccine in two countries, oral cholera vaccine in five countries, and Tetanus-diphtheria (Td) vaccine in seven countries [5]. The Global Polio Eradication Initiative recommended suspending polio vaccination campaigns until the second half of 2020 [6]. Following the suspension of these immunisation activities, there has been a new polio outbreak in Niger [7]. In Pakistan and Afghanistan, wild poliovirus Type 1 have been reported, and cases of Type 2 poliovirus, mutated from the oral vaccine, have appeared in Chad, Ethiopia, Ghana, and Pakistan [8].
In the COVID-19 pandemic, over 117 million children in 37 countries may miss out on the measles vaccine; while the measles Immunisation campaigns in 24 countries have already been delayed [9]. By the end of 2020, 178 million people are at risk of skipping measles vaccines, according to the Measles and Rubella Initiative, even as measles flares across the globe, including in Afghanistan, Brazil, Cambodia, the Central African Republic, Iraq, Kazakhstan, Nepal, Nigeria, and Uzbekistan [10].
Administering timely vaccines may be affected by factors like natural disasters, which lead to infectious disease outbreaks [11]. Immunisation coverage decreased more than 25% during the Ebola epidemic in West Africa during 2016 [12]. United Nations Children’s Fund (UNICEF) reported that, about 2000 people died of Ebola in the Democratic Republic of Congo, while double of that number died of measles in 2019 because of the disruption of Immunisation services [13].
Thus, the Ministry of Health and Family Welfare (MoHFW) Government of India recommends that childhood vaccinations should be given timeously during the COVID-19 pandemic [14]. The aim of study was to assess the overall trend and to evaluate whether the vaccination coverage has changed during the pandemic.
Materials and Methods
The cross-sectional study was conducted at Jaya Arogya Group of Hospitals (JAH) which is a government sector tertiary care hospital associated with Gajra Raja Medical College, Gwalior, Madhya Pradesh, India. It provides wide range of services from super-specialty care to routine services including Immunisation. The duration of the study was from 1st February 2020 to 31st August 2020. The present study utilised data collected from the patient record available from the Out Patient Department (OPD) register.
Inclusion and Exclusion criteria: All the children who visited the Immunisation OPD during the study period were included to show the trend of vaccination. Thus, a total of 817 children were included in the study. The OPD was closed in April 2020 due to lockdown so no data was available for the April month hence these children were excluded.
Purposive sampling method was used to collect the data. A written permission (I.No.596-A/PSM/2020/16/09/2020) was obtained from the Head of the Department, Preventive and Social Medicine, Gajra Raja Medical college Gwalior, India, for collection of data and to conduct the study.
There is no standard definition available for delayed vaccination, hence considering other studies [15,16], operational definition for “Delayed vaccination” for each vaccine was defined as administration of the vaccine dose after two weeks of the minimum age recommended by the national Immunisation schedule in India.
In the study, Penta 1 series included: Pentavalent-1, IPV-1 (Inactivated Polio Vaccine), PCV-1(Pneumococcal Conjugate Vaccine), OPV-1 (Oral Polio Vaccine-1) and Rotavirus-1. Penta-2 series included: Pentavalent-2, Rotavirus-2 and OPV-2. Penta-3 series includes: Pentavalent-3, OPV-3, Rotavirus-3, PCV-2 and IPV-2. MR-1 includes (Measles Rubella). Booster-I included: MR-2, Diphtheria Pertussis Tetanus (DPT) booster-1 and OPV-booster dose and Booster-II includes DPT-2 and OPV booster dose.
Statistical Analysis
The collected data was coded and entered into Microsoft Excel version-2007. Descriptive statistics (frequency, percentage, mean and standard deviation) were calculated. Chi-square test was applied to test association. To show the time trend, a line diagram was used. The p-value was calculated at 5% level of significance.
Results
The mean age of children was 7.46 months (±13.60). The median age was 2.50 months (1.5-4.5). Among the 817 children that were included, male children were 61.1% (499) while females 38.9% (318).
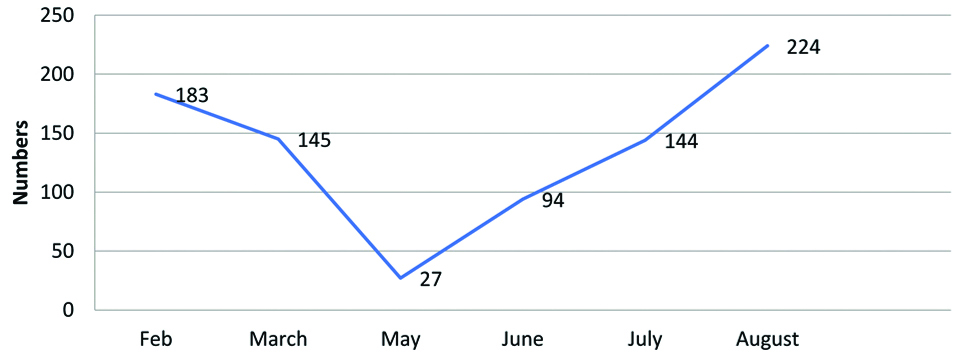
The children vaccinated for BCG, penta-1 series, penta-2 series, penta-3 series, MR-1, Booster-1dose, Booster-II dose were 10.6%, 36.4%, 17.4%, 12.2%, 6.5%, 11.8% and 5.1%, respectively [Table/Fig-1]. In the month of February 22.4% children came for vaccination. From month of March, Immunisation coverage declined from 17.75% to 11.51% in June. It was also seen that after the month of February the number of children for vaccination declined by a great extent reaching to its lowest count i.e., 27 in the month of May and regaining its peak in August [Table/Fig-2]. In the age group 0-2 months and 2-4 months, maximum participants reported for Immunisation i.e., 43.3% and 31.7%, respectively.
Cross-tabulation of vaccination with gender, age, and month.
| Vaccine* | 0 | 1 | 2 | 3 | 4 | 5 | 6 | Total |
|---|
| Gender | Male | 56 (11.2) | 177 (35.5) | 92 (18.5) | 68 (13.6) | 29 (5.8) | 50 (10.0) | 27 (5.4) | 499 |
| Female | 31 (9.8) | 120 (37.7) | 50 (15.7) | 32 (10.1) | 24 (7.5) | 46 (14.5) | 15 (4.7) | 318 |
| Age group (in month) | 0-2 | 87 (24.6) | 267 (75.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 354 |
| 2-4 | 0 (0.0) | 30 (11.6) | 136 (52.5) | 93 (35.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 259 |
| 4-6 | 0 (0.0) | 1 (11.1) | 3 (33.3) | 5 (55.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 |
| 6-12 | 0 (0.0) | 1 (1.9) | 2 (3.7) | 1 (1.9) | 50 (92.5) | 0 (0.0) | 0 (0.0) | 54 |
| >12 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 2 (1.4) | 96 (68.1) | 42 (29.8) | 141 |
| Month | February | 47 (25.7) | 66 (36.1) | 26 (14.2) | 20 (10.9) | 5 (2.7) | 13 (7.1) | 6 (3.3) | 183 |
| March | 38 (26.2) | 62 (42.8) | 17 (11.7) | 6 (4.2) | 7 (4.8) | 7 (4.8) | 8 (5.5) | 145 |
| May | 1 (3.7) | 21 (77.8) | 2 (7.4) | 2 (7.4) | 0 (0.0) | 1 (3.7) | 0 (0.0) | 27 |
| June | 0 (0.0) | 47 (50.0) | 26 (27.7) | 5 (5.3) | 1 (1.0) | 12 (12.8) | 3 (3.2) | 94 |
| July | 1 (0.7) | 48 (33.3) | 37 (25.7) | 29 (20.1) | 8 (5.6) | 15 (10.4) | 6 (4.2) | 144 |
| August | 0 (0.0) | 53 (23.7) | 34 (15.2) | 38 (16.9) | 32 (14.3) | 48 (21.4) | 19 (8.5) | 224 |
| Total | 87 (10.6) | 297 (36.4) | 142 (17.4) | 100 (12.2) | 53 (6.5) | 96 (11.8) | 42 (5.1) | 817 |
*0-BCG, 1-Penta-1 series, 2-Penta-2 series, 3-Penta-3 series, 4-MR, 5-Booster-1 and 6-Booster-II
Monthly distribution of vaccination of children.
Among males, the maximum number received the Penta-1 series vaccine (35.5%), while the maximum number of the female child also received the same (37.7%). Minimum number of children reported for vaccination of Booster-II dose. In the 0-2 month age group, 75.4% children came for Penta-1 series vaccination. In the month of February, vaccination for BCG, Penta-1, Penta-2, Penta-3, MR-1, Booster-I and Booster-II were 25.7%, 36.1%, 14.2%, 10.9%, 2.7%, 7.1% and 3.3%, respectively. Similarly, for the rest of the months, percentage distribution for different vaccines is shown in [Table/Fig-1].
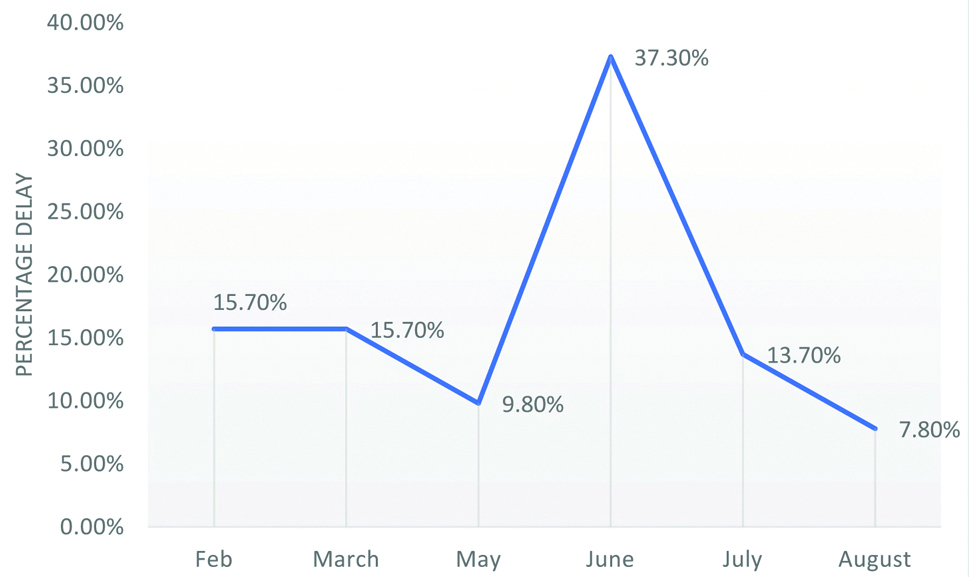
In this study, total of 51 (6.2%) children were found to be delayed for vaccination. Among males, the delay was seen in 7.2% while in females delay was 4.7%. In the age group of 0-2 months, there was no delay while the maximum delay was seen in 4-6 months of age group. Delay in vaccination was found to be significantly distributed in age groups. Children who were delayed for vaccination received Penta-1 series-31 (10.4%), Penta-2 series-18 (12.7%), Penta-3 series-01 (1.0%), MR-1-01 (1.9%). Vaccination, for which children were scheduled was significantly associated with the delay. The maximum monthly delay was seen in May and June which was 18.5% and 20.2%, respectively, while the minimum delay was seen in August (1.8%) [Table/Fig-3].
Association table for variables and delay in vaccination.
| Variables | Delayed in vaccination | Total | χ2, p-value |
|---|
| Yes | No |
|---|
| Gender | Female | 15 (4.7%) | 303 (95.3%) | 318 | 2.07, 0.182 |
| Male | 36 (7.2%) | 463 (92.8%) | 499 |
| Age group (in month) | 0-2 | 0 (0.0%) | 354 (100.0) | 354 | 91.615, <0.001 |
| 2-4 | 42 (16.2%) | 217 (83.8) | 259 |
| 4-6 | 4 (44.4%) | 5 (55.6%) | 9 |
| 6-12 | 3 (5.6%) | 51 (94.4%) | 54 |
| >12 | 2 (1.4%) | 139 (98.6%) | 141 |
| Vaccination* | 0 | 0 (0.0%) | 87 (100%) | 87 | 40.369, <0.001 |
| 1 | 31 (10.4%) | 266 (89.6%) | 297 |
| 2 | 18 (12.7%) | 124 (87.3%) | 142 |
| 3 | 1 (1.0%) | 99 (99.0%) | 100 |
| 4 | 1 (1.9%) | 52 (98.1%) | 53 |
| 5 | 0 (0.0%) | 96 (100.0%) | 96 |
| 6 | 0 (0.0%) | 42 (100.0%) | 42 |
| Month | February | 8 (4.4%) | 175 (95.6%) | 183 | 47.595, <0.001 |
| March | 8 (5.5%) | 137 (94.5%) | 145 |
| May | 5 (18.5%) | 22 (81.5%) | 27 |
| June | 19 (20.2%) | 75 (79.8%) | 94 |
| July | 7 (4.9%) | 137 (95.1%) | 144 |
| August | 4 (1.8%) | 220 (98.2%) | 224 |
| Total | 51 (6.2%) | 766 (93.8%) | 817 | |
*0-BCG, 1-Penta 1 series, 2-Penta 2 series, 3-Penta 3 series, 4-MR, 5-Booster-I and 6-Booster-II, χ2: Chi-square; p-value <0.05 considered significant
The delay that was seen for the Penta-2 series, Penta 3 series, and MR-1 was 43.1%, 51.0% and 5.9% respectively and the difference was statistically significant (p=0.005) [Table/Fig-4]. Monthly distribution for the delay in vaccination showed that June witnessed the highest number of delay (37.30%) [Table/Fig-5].
Association between the vaccine and delay in vaccination.
| Vaccine | Delayed n (%) | Not-delayed n (%) | χ2/p-value |
|---|
| Penta 2 series | 22 (43.1) | 120 (49.2) | 10.639/0.005 |
| Penta 3 series | 26 (51.0) | 74 (30.3) |
| MR 1 | 3 (5.9) | 50 (20.5) |
| Total | 51 | 244 |
p-value <0.05 considered significant
Monthly distribution of delayed children.
Discussion
Recent reports worldwide suggest that routine childhood Immunisation coverage might have decreased during the COVID-19 pandemic [17,18]. In this study, it was seen that total 817 children came for Immunisation during 6 months. The number of children in the month of February was 22.4% which started declining in the subsequent months, reaching its lowest in the month of May i.e., 3.3%. Causes of this decrease/interrupted Immunisations are due to parents’ fear, restrictions of movement/lockdown policies, changing priorities for COVID-19 among health-care personnel and logistics delivery issues (i.e., vaccine transport delays) [5,19]. The World Health Organisation (WHO), UNICEF, and Global Alliance for Vaccines and Immunisation (GAVI), the Vaccine Alliance have reported that routine Immunisation programs have been substantially disrupted in at least 68 countries, affecting around 80 million children. Of the 129 countries in which data were available, more than half reported moderate to severe disruptions or total suspension of vaccination services during March-April 2020 [5]. There was a full cessation of routine Immunisations from April 1-15,2020 in Vietnam. In Pakistan, polio catch-up Immunisation campaigns were postponed until June 1st, 2020 [20]. The situation is quite similar to this study where Immunisation OPD was closed in April due to total lockdown.
A decline in the routine Immunisation of about 14.4% was observed in this study during the initial days of lockdown (i.e., from March to May) which is consistent with a study in England where the number of Measles, Mumps, Rubella (MMR) vaccine delivered fell by 20% during the first 3 weeks of the lockdown [21] and similarly in Indonesia about 19.7% drop in MMR vaccination in April 2020 was seen [22].
Out of all the children, majority received Pentavalent-1 series vaccine (36.4%), this is due to the fact that the percentage of children in the age group of 0-2 months was also maximum (43.3%). Number of children both male and female receiving DPT-2 booster vaccine was minimum, similar findings were seen in a study done by Singh J and Neki NS, where number of children receiving DPT-2 booster vaccine was minimum (9.27%) [23].
Another important observation in this study was delayed vaccination which was found in 6.2% children. This could be due to lockdown policies implemented by the government during initial phase of COVID-19 and fear of parents about potentially exposing their children to COVID-19 during routine follow-up visits [5]. Delay in vaccination is significantly associated with monthly distribution of the children reporting for vaccination. Maximum delay was seen in the month of June (20.2%) followed by month of May (18.5%). The possible reason may be due to closed Immunisation OPD in April that led to piling up of children due for vaccination and starting of the unlock-1 period from 1st June 2020 which led to a comparative rise in the number of children coming to immunisation OPD. The minimum delay was seen in August (1.8%) as the number of children coming for vaccination started increasing.
In fact, the number of children vaccinated in August was maximum (224) when compared to other months. This shows that routine childhood Immunisation services came back to its normal pace after a sudden dip during the lockdown phase. A similar pattern of routine childhood immunisation was seen in a study done in the UK by McDonald HI et al., [21] wherein the 3 weeks after the introduction of full physical distancing measures (weeks 13 to 15), hexavalent vaccination was 6.7% lower and MMR vaccination 19.8% lower than in 2019, although physical distancing measures remained unchanged nationally throughout the rest of the study period, vaccination counts were higher in weeks 16 and 17 of the year 2020 than for the same weeks in 2019 for both hexavalent and MMR vaccines. If we look at the vaccine-specific delay, maximum delay was seen for pentavalent 3 series and pentavalent 2 series vaccines as most of the children delayed for vaccination was in the age group of 2-4 months while no delay was seen for BCG and pentavalent 1 series vaccine because whenever a child below 1 year of age who is delayed for age-specific vaccination came to OPD, BCG and Penta 1 series vaccine is given to the child irrespective of his/her age-specific due vaccine status. Since this study was conducted in a tertiary care hospital so 100% of newborn children would receive birth dose vaccination and this again added to a 0% delay in BCG vaccination. It seems that the delay observed was a planned one rather than lack of adherence to routine immunisation program. One plausible explanation is that covid-19 messaging about staying home initially overwhelmed the message that the Immunisation program was to remain operating as usual [21].
Limitation(s)
There was no direct contact with the participants or the care givers so it was not possible to incorporate all the socio-demographic variables of the study participants. The data was taken from the OPD register hence any possible erroneous entries made at the time of vaccination could not be ruled out.
Conclusion(s)
The vaccination during the lockdown phase of the COVID-19 pandemic had declined but after June 2020 vaccination again started showing a positive upward trend which is critical to maintaining momentum on routine childhood Immunisation. The study recommends that to prevent overcrowding, immunisation sessions should be conducted with fixed prior appointments while ensuring health workers’ safety. Catch-up campaigns should be organised with all necessary safety precautions. Vaccine timeliness should be given utmost priority, focused strategies to be implemented to achieve a significant and sustainable increase in vaccinations during pandemics.
*0-BCG, 1-Penta-1 series, 2-Penta-2 series, 3-Penta-3 series, 4-MR, 5-Booster-1 and 6-Booster-II
*0-BCG, 1-Penta 1 series, 2-Penta 2 series, 3-Penta 3 series, 4-MR, 5-Booster-I and 6-Booster-II, χ2: Chi-square; p-value <0.05 considered significant
p-value <0.05 considered significant
[1]. World Health Organisation. Timeline of WHO’s response to COVID-19. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline. (accessed on 20 September 2020) [Google Scholar]
[2]. World Health Organisation. WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int. (accessed on 05 January 2021) [Google Scholar]
[3]. Dinleyici EC, Borrow R, Safadi MAP, van Damme P, Munoz FM, Vaccines and routine immunization strategies during the COVID-19 pandemicHum Vaccin Immunother 2021 17(2):400-07.10.1080/21645515.2020.180477632845739 [Google Scholar] [CrossRef] [PubMed]
[4]. Ozawa S, Clark S, Portnoy A, Grewal S, Brenzel L, Walker DG, Return on investment from childhood Immunisation in low-and middle-income countries, 2011-20Health Aff 2016 35(2):199-207.10.1377/hlthaff.2015.108626858370 [Google Scholar] [CrossRef] [PubMed]
[5]. World Health Organisation. At least 80 million children under one at risk of diseases such as diphtheria, measles and polio as COVID-19 disrupts routine vaccination efforts warn GAVI, WHO, and UNICEF. Available at: https://www.who.int/news-room/detail/22-05-2020-at-least-80-million-children-under-one-at-risk-of-diseases-such-as-diphtheria-measles-and-polio-as-covid-19-disrupts-routine-vaccination-efforts-warn-gavi-who-and-unicef (accessed on 13 September 2020) [Google Scholar]
[6]. Roberts L, Global polio eradication falters in the final stretchScience 2020 367(6473):14-15.Doi: 10.1126/science.367.6473.1410.1126/science.367.6473.1431896700 [Google Scholar] [CrossRef] [PubMed]
[7]. World Health Organisation Africa. Niger reports new polio outbreak. Available at: https://www.afro.who.int/news/niger-reports-new-polio-outbreak (accessed on 03 January 2021) [Google Scholar]
[8]. Global Polio Eradication Initiative. Weekly country updates as of 30 December 2020. Available at: http://polioeradication.org/polio-today/polio-now/this-week/ (accessed on 03 January 2021) [Google Scholar]
[9]. World Health Organisation. More than 117 million children at risk of missing out on measles vaccines, as COVID-19 surges. Available at: https://www.who.int/Immunisation/diseases/measles/statement_missing_measles_vaccines_covid-19/en/ (accessed on 03 January 2021) [Google Scholar]
[10]. Hoffman J, Maclean R, Slowing the Coronavirus is speeding the spread of other diseasesNew York Times 14 Jun 2020 Available at: https://www.nytimes.com/2020/06/14/health/coronavirus-vaccines-measles.html (accessed on 03 January 2021) [Google Scholar]
[11]. Kang E, Impact of Disasters on Community Medical Screening Examination and Vaccination Rates: The Case of the Sewol Ferry Disaster in Ansan, KoreaDisaster Med Public Health Prep 2020 4(3):01-06.10.1017/dmp.2020.2932241311 [Google Scholar] [CrossRef] [PubMed]
[12]. Sun X, Samba TT, Yao J, Yin W, Xiao L, Liu F, Impact of the Ebola outbreak on routine Immunisation in western area, Sierra Leone-A field survey from an Ebola epidemic areaBMC Public Health 2017 17(1):01-06.10.1186/s12889-017-4242-728446173 [Google Scholar] [CrossRef] [PubMed]
[13]. United Nations News. Measles claims more than twice as many lives than Ebola in DR Congo. 27 November 2019. Available at: https://news.un.org/en/story/2019/11/1052321 (accessed on 03 January 2021) [Google Scholar]
[14]. MOHFW. Immunisation Services during and post COVID-19 Outbreak. Available at: https://www.mohfw.gov.in/pdf/3ImmunisationServicesduringCOVIDOutbreakSummary150520202.pdf (accessed on 11 November 2020) [Google Scholar]
[15]. Yadav K, Srivastava R, Kumar R, Chinnakal P, Rai SK, Krishnan A, Significant vaccination delay can occur even in a community with very high vaccination coverage: Evidence from Ballabgarh, IndiaJournal of Tropical Pediatrics 2012 58(2):133-38.10.1093/tropej/fmr05921742766 [Google Scholar] [CrossRef] [PubMed]
[16]. Clark A, Sanderson C, Timing of children’s vaccinations in 45 low-income and middle-income countries: An analysis of survey dataThe Lancet 2009 373(9674):1543-49.10.1016/S0140-6736(09)60317-2 [Google Scholar] [CrossRef]
[17]. Santoli JM, Lindley MC, DeSilva MB, Kharbanda EO, Daley MF, Galloway L, Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration-United States, 2020MMWR Morb Mortal Wkly Rep 2020 69:591-93.10.15585/mmwr.mm6919e232407298 [Google Scholar] [CrossRef] [PubMed]
[18]. Bramer CA, Kimmins LM, Swanson R, Kuo J, Vranesich P, Jacques-Carroll LA, Decline in child vaccination coverage during the COVID-19 pandemic-Michigan Care Improvement RegistryMMWR Morb Mortal Wkly Rep 2020 69(20):630-31.10.15585/mmwr.mm6920e132437340 [Google Scholar] [CrossRef] [PubMed]
[19]. Saso A, Skirrow H, Kampmann B, Impact of COVID-19 on immunisation services for maternal and infant vaccines: Results of a survey conducted by imprint-The immunising pregnant women and infants networkVaccines 2020 8(3):55610.3390/vaccines803055632972015 [Google Scholar] [CrossRef] [PubMed]
[20]. Nelson R, COVID-19 Disrupts Vaccine DeliveryLancet Infect Dis 2020 20(5):54610.1016/S1473-3099(20)30304-2 [Google Scholar] [CrossRef]
[21]. McDonald HI, Tessier E, White JM, Woodruff M, Knowles C, Bates C, Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020Eurosurveillance 2020 25(19):200084810.2807/1560-7917.ES.2020.25.19.200084832431288 [Google Scholar] [CrossRef] [PubMed]
[22]. Jakarta Globe. Immunisation Should Continue Amid Pandemic: Health Ministry. Available at: https://jakartaglobe.id/news/Immunisation-should-continue-amid-pandemic-health-ministry/. (accessed on 01 December 2020) [Google Scholar]
[23]. Singh J, Neki NS, Evaluation of vaccination coverage and Dropout rates among children of age 0-5 years in slums of Amritsar cityInt J Curr Res Biol Med 2018 3(3):16-22. [Google Scholar]