Severe Acute Acrocyanosis and Digital Gangrene as a Sign of Catastrophic COVID-19 Infection
Arun Agarwal1, Ambika Sharma2, Rekha Jakhar3, Mudit Agarwal4
1 Director and Head, Department of Internal Medicine, Fortis Escorts Hospital, Jaipur, Rajasthan, India.
2 Resident, Department of Internal Medicine, Fortis Escorts Hospital, Jaipur, Rajasthan, India.
3 Resident, Department of Internal Medicine, Fortis Escorts Hospital, Jaipur, Rajasthan, India.
4 Intern, Department of Internal Medicine, AIIMS, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Arun Agarwal, A-235, Malviya Nagar, Jaipur, Rajasthan, India.
E-mail: mpicdrarun@gmail.com
Coronavirus Infection Disease-2019 (COVID-19) may present with different symptoms and complications during its course. Emerging evidence suggests that it induces a hypercoagulable state with micro and macroangiopathy. This hypercoagulopathy has been identified in a subset of critically ill COVID-19 patients. However, extremity ischaemia with acrocyanosis and digital gangrene has not been commonly reported with COVID-19. It is caused due to microangiopathic and immunothrombosis phenomenon, and may be accompanied by microvascular involvement of other organs. Here, a case of critically ill 67-year-old male COVID-19 patient is reported who developed digital acrocyanosis and gangrene in lower limbs while being mechanically ventilated for severe Acute Respiratory Distress Syndrome (ARDS) despite being haemodynamically stable (i.e., not needing vasopressor) and on therapeutic anticoagulation. He subsequently succumbed to his disease due to multiorgan dysfunction. This suggests that extremity ischaemia correlates with poor prognosis in this small subset of critically ill COVID-19 patients, and can have a prognostic role in the disease outcome. It may be the first clinical manifestation even in non-vasculopathic patients.
Cytokine storm, Hypercoagulopathy, Immunothrombosis, Microangiopathy
Case Report
A 67-year-old male presented to the Emergency Department on 26th September 2020. He had history of fevers, chills, breathlessness and weakness in the past one week. He took treatment locally after testing positive for COVID-19 at an outside hospital on 21st September 2020. He had a history of type 2 diabetes mellitus, hypertension and Koch’s chest 4 years back. His glycaemia and hypertension were controlled on treatment.
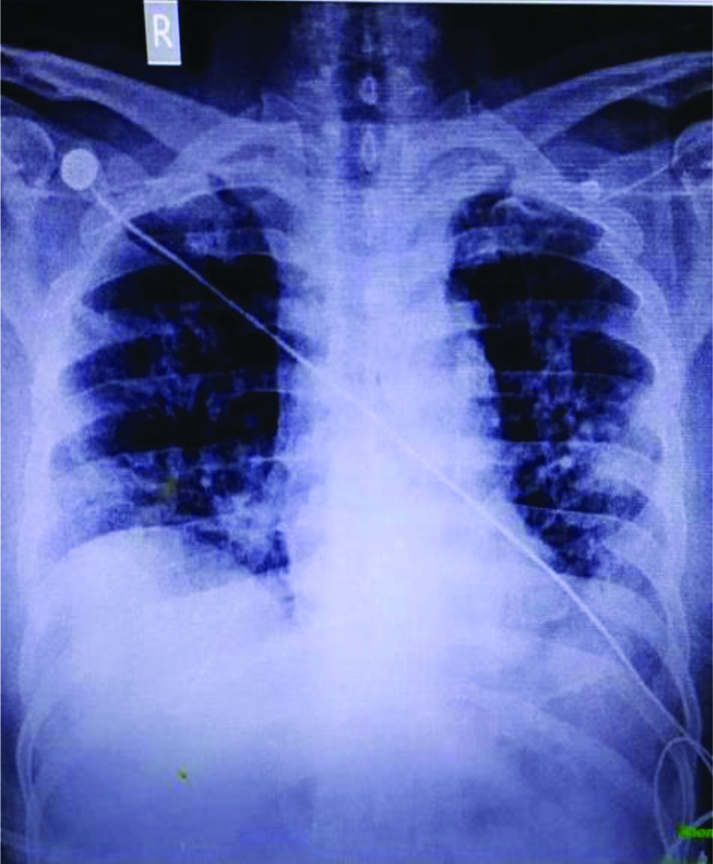
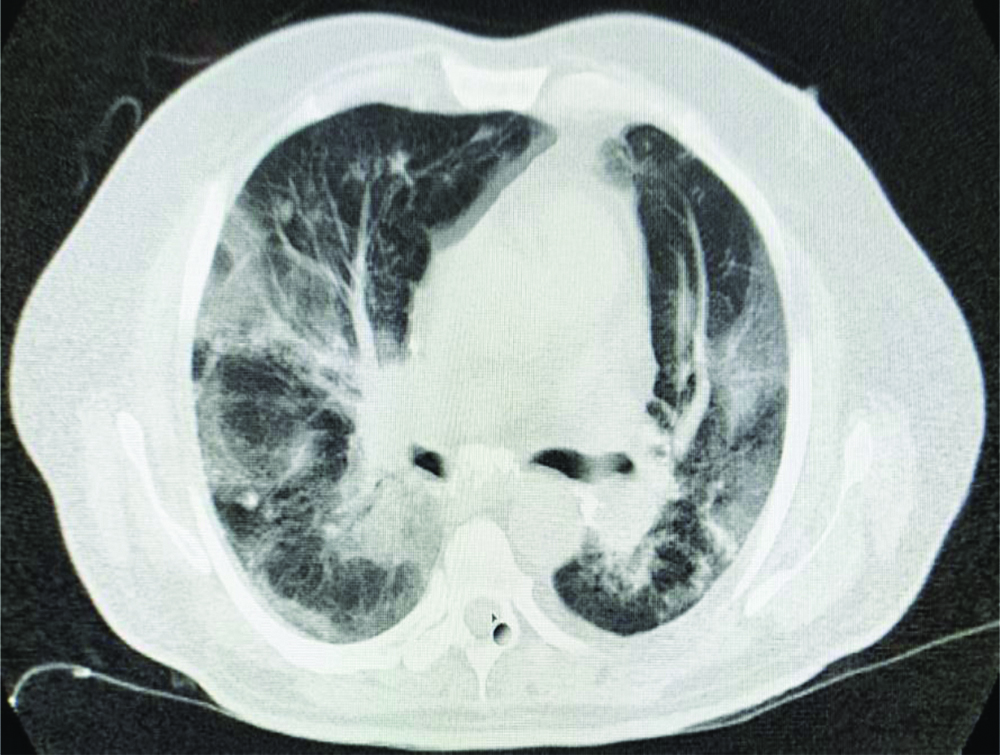
The patient presented to the hospital because of worsening of the symptoms. Upon arrival in triage, his vital signs included a temperature of 98.40F, blood pressure of 137/78 mmHg, pulse of 87 beats/min, respiratory rate of 24 breaths/min, and oxygen saturation of 98% on 5 liters per minute of oxygen with face mask. Systemic examination showed presence of bilateral coarse/fine crepitations over infrascapular and lower axillary areas and non tender mild hepatomegaly. Rest of the clinical examination was essentially normal. His Arterial Blood Gas (ABG) showed pH 7.369, partial pressure of Carbon Dioxide (pCO2) 32.7 mmHg, partial pressure of Oxygen (pO2) was 66.3 mmHg, Bicarbonate (HCO3) (act) was 18.4 mmol/L consistent with chronic respiratory alkalosis. PaO2/Fraction of Inspired Oxygen (FiO2) ratio was 165 suggesting moderate ARDS. The initial chest X-ray demonstrated bibasal pneumonitis [Table/Fig-1]. His High Resolution Computed Tomography (HRCT) chest done on 26/09/20 was suggestive of bilateral interstitial pneumonia based on CO-RAD 6 (The corona virus disease-2019 Reporting and Data System [1], with Cycle Threshold (CT) severity score of 12/25 (moderate disease) along with old healed fibrotic Koch’s lesion [Table/Fig-2].
X-ray Chest of the patient dated 26/09/2020.
Axial view of HRCT chest.
He was subsequently admitted to the COVID-19 Intensive Care Unit (ICU) for management of acute hypoxemic respiratory failure owing to COVID-19 ARDS. The patient was managed with antibiotics, remdesivir, fractionated heparin (Enoxaparin) 60 mg twice daily subcutaneously, insulin, dexamethasone, and also received 200 mL COVID convalescent Plasma (CCP). However, his oxygen requirement increased over next 2-3 days and he developed persistent hypoxemia despite 100% FiO2, 60 Liters of oxygen through High Flow Nasal Cannula (HFNC). He was later intubated and put on mechanical ventilation on 29/09/20. On 30/09/2020 he had a trial fibrillation with rapid ventricular response was detected during monitoring and was controlled on antiarrhythmic therapy with cordarone. He also developed acute kidney injury and was managed medically. The patient did not require renal replacement therapy. Written consent was obtained from the guardian of the patient.
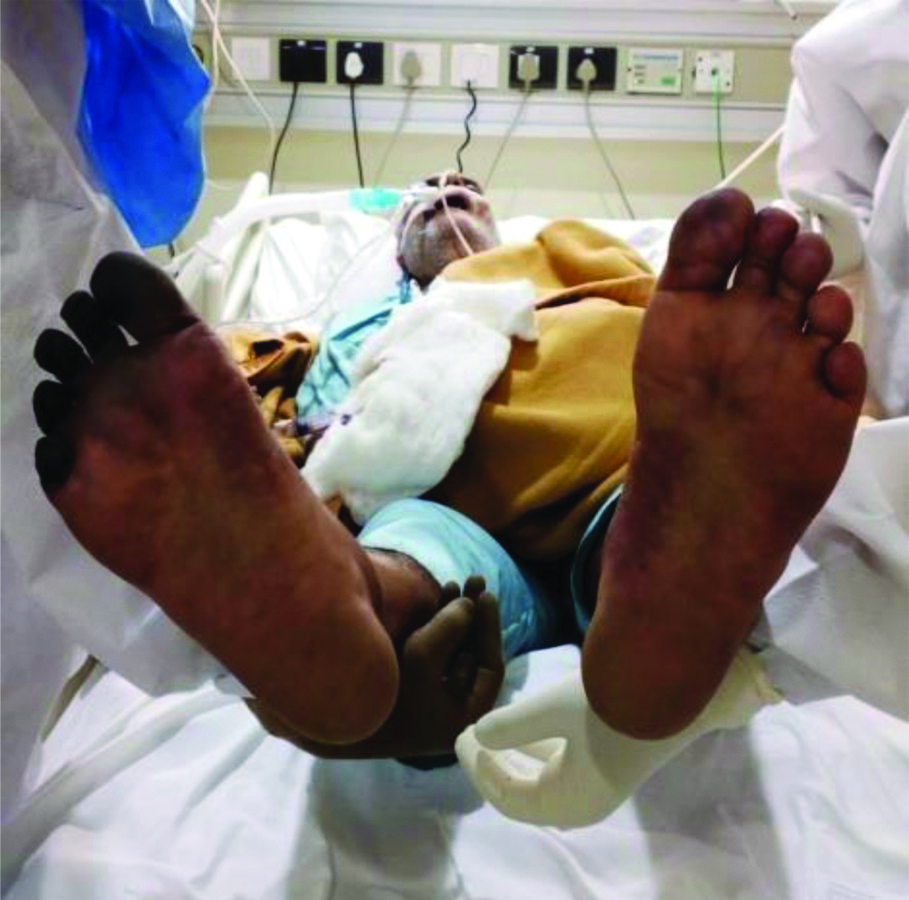
On 30th September 2020, approximately five days after presentation to the triage (and 12 days from symptom onset), the patient developed gradually worsening duskiness of all toes of the right feet and first four toes of the left feet along with some patchy areas of duskiness on the ventral surface of left foot [Table/Fig-3,4]. He had no arterial lines placed in femoral arteries and was haemodynamically stable till this time. The physical examination showed a bluish black appearance of these toes, nail beds and few patchy areas in left feet on ventral aspect. Palpation using finger tip observed the cool skin and all peripheral arterial pulses were palpable. Colour doppler and pulse wave doppler imaging of foot arteries was normal.
Bluish black discolouration over first four toes and patchy areas over sole of left foot.
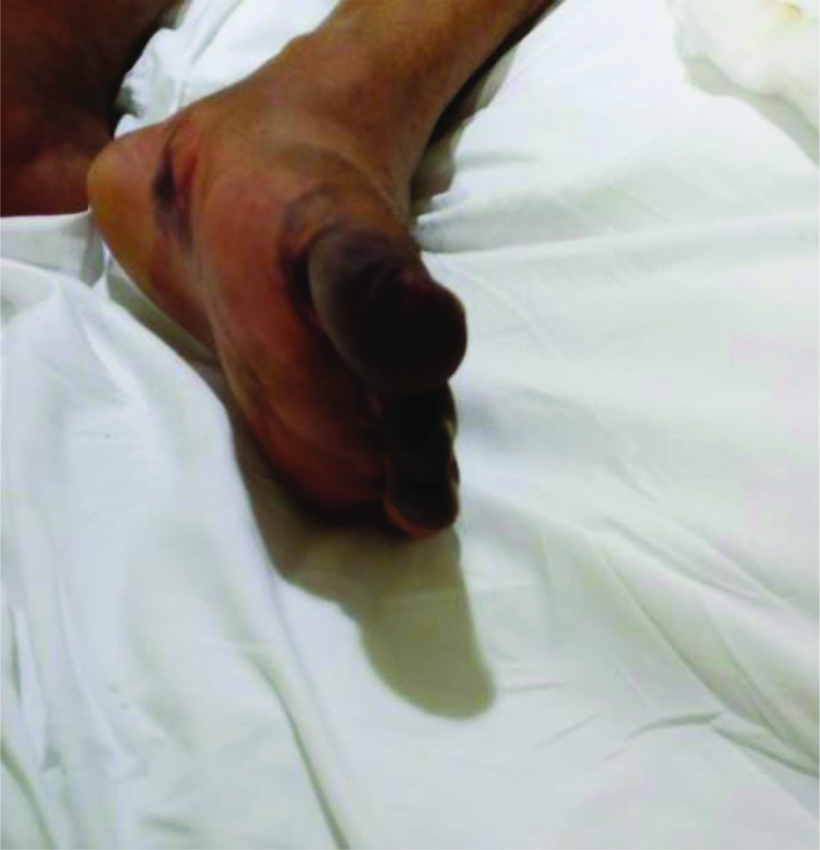
Bilateral colour changes over both feets
At this time his inflammatory markers also showed marked increase with serum ferritin of >10000 ng/mL, C-Reactive Protein (CRP) 218.6 mg/L, Lactate Dehydrogenase (LDH) 639 U/L and D-Dimer 7090 ng/mL, suggesting Cytokine storm/Hyper-inflammation syndrome. The laboratory values are tabulated in [Table/Fig-5]. His urine culture grew Candida albicans and blood cultures were sterile.
| Test | Normal range | 26th September 2020 | 28th September 2020 | 30th September 2020 | 1st October 2020 | 3rd October 2020 | 5th October 2020 |
|---|
| ESR (mm/hr) | 0-14 | 60 | | | 22 | | |
| CRP (mg/L) | 0-5 | 114.4 | 143 | 218.6 | 35.4 | 86.7 | 151.6 |
| LDH (U/L) | 135-225 | 427 | 415 | 639 | | 422 | |
| D DIMER (ng/mL) | <255 | 287 | 275 | 7090 | | 4660 | 1180 |
| IL 6 (pg/mL) | <7 | 26.5 | | | | |
| Ferritin (ng/mL) | 30-400 | 1002 | 1093 | 10230 | 409.6 | 1497 | >20000 |
| Creatinine (mg/dL) | 0.7-1.2 | 1.16 | 1.15 | 1.85 | 1.39 | 2.88 | 3.14 |
| Hb (gm/dL) | 13-17 | 14.2 | | 16.6 | | 12.2 | 12.4 |
| TLC (103/uL) | 4-10 | 14.1 | | 27.7 | 24.7 | 17.1 | 21.1 |
| Platelets (103/UL | 150-410 | 391 | | 507 | | 164 | 130 |
| AST/ALT (U/L) | <40/ <41 | 33/37 | 25/21 | 18/16 | | | |
| Procalcitonin (ng/mL) | <0.046 | 0.695 | | 1.050 | | 0.439 | |
| PRO BNP (pg/mL) | <125 | 1176 | | | | | |
| Fibrinogen (mg/dL) | 200-400 | | | | | 419 | |
| CTSS | x | 12/25 | | | | | |
ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; LDH: Lactate dehydrogenase; IL-6: Interleukin-6; Hb: Haemoglobin; TLC: Total leukocyte count; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; PRO-BNP: PRO-B-Type natriuretic peptide; CTSS: CT severity scoring system; mm: Millimeter; hr: Hour; mg: Milligram; U: Unit; L: Litre; uL: Microlitre; ng: Nanogram; zpg: Picogram; dL: Deciliter; IU: International unit
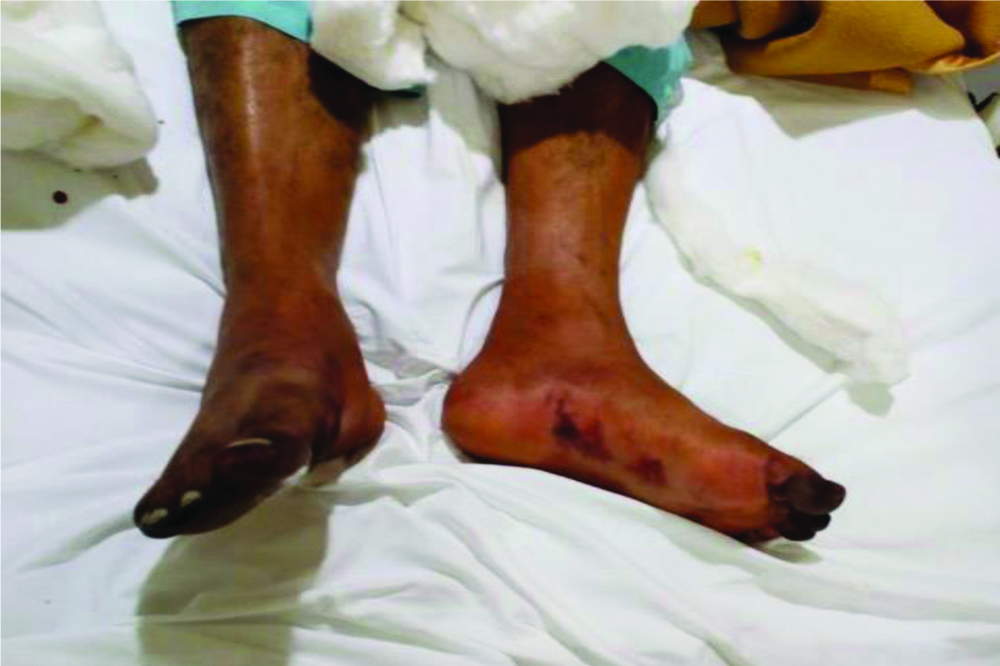
Based on this presentation, he was started on Unfractionated heparin with initial bolus of 80 units/kg, followed by a continuous infusion of 12 to 18 units/kg/hour. Infusion rate was adjusted to maintain anticoagulation target based as per institutional protocol. Along with this thermal warming of the affected limbs and application of topical nitroglycerin to the affected area was also recommended. The hyper-inflammation syndrome was treated with intravenous methyl prednisolone 1 gram pulse therapy for 3 days and IV IG (Intravenous Immunoglobulin) in dose of 400 mg/ kg/day for 5 days with near complete recovery in left feet in next 72 hours [Table/Fig-6]. However, he developed septic shock along with multiorgan dysfunction. His condition worsened on 05/10/2020 and the patient died of multiorgan failure on 07/10/20.
Significant recovery of left feet acrocyanosis with persistent colour changes and development of dry gangrene in right feet on 03/10/20.
Discussion
Although the disease COVID-19 is known predominantly for its respiratory manifestations, it can present with many symptoms and complications ranging from flu-like symptoms to ARDS (also known as COVID disease associated ARDS or CARDS), coagulation dysfunction, septic shock, and multiorgan failure, besides being asymptomatic in certain subset of patients [2,3]. A subset of critically ill patients demonstrates clinically notable hypercoagulability. Many of these symptoms or complications are caused by cytokines and mediators of the immune system induced by the infection along with Cytokine Storm Syndrome (CSS) in some patients [2,3]. CSS is the result of the overproduction of major proinflammatory cytokines such as Tumour Necrosis Factor-α (TNF-α), IL-6, and IL-1β that can produce hypercoagulable state and multiple organ dysfunctions [2,3].
This hyper inflammatory condition or CRS leads to the phenomenon of immunothrombosis which is a result of endovascular damage, increased platelet activity, and over activation of coagulation cascade [4]. Further, this phenomenon of clot formation can be observed in large and small blood vessels, along with in-situ pulmonary thrombosis and also thromboembolism (PTE) [4,5]. Nevertheless, the coagulopathy of COVID-19 and Disseminated Intravascular Coagulation (DIC) as one of the first manifestations of COVID-19 disease has already been reported [6]. Furthermore, acrocyanosis has also been described in critically ill patients with the disease because of hypercoagulability [7-9]. Acral distribution and delayed progression of ischaemia after the acute respiratory insult imply that the thrombotic microangiopathy is secondary to cold sensitive antibody/immunoglobulin activation in response to the virus, rather than a direct viral effect [10]. The index patient also developed acrocyanosis and gangrene after the acute respiratory insult. Recent data suggest that the presence of a complement mediated microvascular injury in multiple organ systems possibly leads to multiorgan dysfunction [10,11].
The case reported here, presented with moderate CARDS and despite adequate management progressed to severe CARDS and was ventilated on day 4 of admission. He developed Cytokine Release Syndrome (CRS) on day 5 with marked elevation in his inflammatory markers. Certain evidence shows that, during the COVID-19 epidemic, the severe deterioration of some patients has been closely related to the cytokine storm in their bodies [12]. The development of acrocyanosis in bilateral distal feet and sole and progression to digital gangrene coincided with the peaking of inflammatory markers, further suggesting underlying patho-physiology involving hyper inflammation, hypercoagulability, immunothrombosis and CRS. Progression of ischaemia despite preserved major peripheral pulses and normal arterial doppler studies in the patient suggests a micro vascular aetiology to the digital acrocyanosis and gangrene. He was managed with unfractionated heparin in recommended dosage, although he had been receiving fractionated heparin since his admission. His left feet did show significant improvement. Progression to gangrene as well as improvement in ischaemia has been described in other case reports also [7,9,13].
Another possible explanation for the ischaemic phenomenon is vasopressor use [14,15]. Catecholamines may play a role in increasing cytokines such as IL-6 which can aggravate the complications of COVID-19 [16]. However, he did not receive any vasopressor by the time he developed digital ischaemia. He was given vasopressor later in the course on day 8. Another possibility is embolic phenomena. Thrombosis resulting from arterial line placement has been reported in literature [17], but our patient did not have any arterial line placed in lower limbs and the digital ischaemia does not fit with this hypothesis. Some authors have suggested antiphospholipid antibodies as a contributor to, or perhaps cause of, such thrombotic events [13,18]. Although, it appears unlikely in the clinical context, they were not tested in the index case and this hypothesis was as a potential explanation for these ischaemic events was acknowledge.
Conclusion(s)
The presence of acrocyanosis in patients with COVID-19 can indicate organ damage due to vascular involvement. It is a marker of severe illness and can have a prognostic role suggesting poor outcomes in these patients. High plasma levels of CRP, IL-6, D-dimer, ferritin and fibrinogen may also represent some important risk markers that increase the chances of microangiopathy. Changing the prophylactic dose to intermediate or therapeutic dose of anticoagulant drugs is recommended in all severe COVID-19 cases, along with adequate vasodilator therapy, in case patient develops acrocyanosis. Surgical intervention is not recommended. A targeted immunotherapy is needed that would effectively manage the catastrophic microangiopathy response induced by COVID-19 disease.
ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; LDH: Lactate dehydrogenase; IL-6: Interleukin-6; Hb: Haemoglobin; TLC: Total leukocyte count; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; PRO-BNP: PRO-B-Type natriuretic peptide; CTSS: CT severity scoring system; mm: Millimeter; hr: Hour; mg: Milligram; U: Unit; L: Litre; uL: Microlitre; ng: Nanogram; zpg: Picogram; dL: Deciliter; IU: International unit
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Dec 19, 2020
Manual Googling: Mar 04, 2021
iThenticate Software: Mar 22, 2021 (23%)
[1]. Prokop M, van Everdingen W, van Rees Vellinga T, Quarles van Ufford H, Stöger L, Beenen L, CO-RADS: A Categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluationRadiology 2020 296(2):E97-104.10.1148/radiol.202020147332339082 [Google Scholar] [CrossRef] [PubMed]
[2]. Pourdowlat G, Naderi Z, Seif F, Mansouri D, Raji H, Acrocyanosis and digital necrosis are associated with poor prognosis in COVID-19Clin Case Rep 2020 8(12):2769-72.10.1002/ccr3.327633363819 [Google Scholar] [CrossRef] [PubMed]
[3]. Jose RJ, Manuel A, COVID-19 cytokine storm: The interplay between inflammation and coagulationLancet Respir Med 2020 8(6):e46-47.10.1016/S2213-2600(20)30216-2 [Google Scholar] [CrossRef]
[4]. Vardon-Bounes F, Ruiz S, Gratacap MP, Garcia C, Payrastre B, Minville V, Platelets are critical key players in sepsisInt J Mol Sci 2019 20(14):349410.3390/ijms2014349431315248 [Google Scholar] [CrossRef] [PubMed]
[5]. Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli F, Thrombotic events in SARS-CoV-2 patients: An urgent call for ultrasound screeningIntensive Care Med 2020 46(6):1121-23.10.1007/s00134-020-06040-332322918 [Google Scholar] [CrossRef] [PubMed]
[6]. Novara E, Molinaro E, Benedetti I, Bonometti R, Lauritano EC, Boverio R, Severe acute dried gangrene in COVID-19 infection: A case reportEur Rev Med Pharmacol Sci 2020 24(10):5769-71. [Google Scholar]
[7]. Zhang Y, Cao W, Xiao M, Li YJ, Yang Y, Zhao J, Clinical and coagulation characteristics of seven patients with critical COVID-2019 pneumonia and acro-ischemiaZhonghua Xue Ye Xue Za Zhi Zhonghua Xueyexue Zazhi 2020 41(0):E006 [Google Scholar]
[8]. Qian SZ, Pan JY, COVID-19 With Limb Ischemic NecrosisJ Cardiothorac Vasc Anesth 2020 34(10):2846-47.10.1053/j.jvca.2020.03.06332359711 [Google Scholar] [CrossRef] [PubMed]
[9]. Schultz K, Wolf JM, Digital Ischemia in COVID-19 Patients: Case ReportJ Hand Surg 2020 45(6):518-22.10.1016/j.jhsa.2020.04.02432387155 [Google Scholar] [CrossRef] [PubMed]
[10]. Wang JS, Pasieka HB, Petronic-Rosic V, Sharif-Askary B, Evans KK, Digital gangrene as a sign of catastrophic coronavirus disease 2019-related MicroangiopathyPlast Reconstr Surg Glob Open [Internet] 2020 Jun 16 [cited 2021 Feb 10] 8(7)Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7413788/10.1097/GOX.000000000000302532802690 [Google Scholar] [CrossRef] [PubMed]
[11]. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five casesTransl Res 2020 220:01-13.10.1016/j.trsl.2020.04.00732299776 [Google Scholar] [CrossRef] [PubMed]
[12]. Ye Q, Wang B, Mao J, The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19J Infect 2020 80(6):607-13.10.1016/j.jinf.2020.03.03732283152 [Google Scholar] [CrossRef] [PubMed]
[13]. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19N Engl J Med 2020 382(17):e3810.1056/NEJMc200757532268022 [Google Scholar] [CrossRef] [PubMed]
[14]. Shin JY, Roh SG, Lee NH, Yang KM, Ischemic necrosis of upper lip, and all fingers and toes after norepinephrine useJ Craniofac Surg 2016 27(2):453-54.10.1097/SCS.000000000000246326854781 [Google Scholar] [CrossRef] [PubMed]
[15]. Simman R, Phavixay L, Bilateral toe necrosis resulting from norepinephrine bitartrate usageAdv Skin Wound Care 2013 26:254-56.10.1097/01.ASW.0000431083.77517.fd23685523 [Google Scholar] [CrossRef] [PubMed]
[16]. Wright DJM, Prevention of the cytokine storm in COVID-19Lancet Infect Dis 2021 21(1):25-26.10.1016/S1473-3099(20)30376-5 [Google Scholar] [CrossRef]
[17]. Paik JJ, Hirpara R, Heller JA, Hummers LK, Wigley FM, Shah AA, Thrombotic complications after radial arterial line placement in systemic sclerosis: A case seriesSemin Arthritis Rheum 2016 46(2):196-99.10.1016/j.semarthrit.2016.03.01527139167 [Google Scholar] [CrossRef] [PubMed]
[18]. El Hasbani G, Taher A T, Jawad A, Uthman I, COVID-19, Antiphospholipid antibodies, and catastrophic antiphospholipid syndrome: A possible association?. Clinical medicine insightsArthritis and musculoskeletal disorders 2020 13SAGE:01-08.10.1177/117954412097866733328777 [Google Scholar] [CrossRef] [PubMed]