COVID-19 and Malaria Co-infection Management in Post Liver Transplant- A Case Report
Saniya Wasim Shaikh1, Sampathkumar Mahadevappa Mahendrakar2, Sulaiman Sadruddin Ladhani3, Azizullah Hafizullah Khan4
1 Postgraduate Trainee in Tropical Medicine and Health, Department of Internal Medicine, Prince Aly Khan Hospital, Mumbai, Maharashtra, India.
2 Consultant Intensivist and Emergency Head, Department of Internal Medicine, Prince Aly Khan Hospital, Mumbai, Maharashtra, India.
3 Head, Department of Chest Medicine, Prince Aly Khan Hospital, Mumbai, Maharashtra, India.
4 Consultant Physician and Intensivist, Department of Internal Medicine, Prince Aly Khan Hospital, Mumbai, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sulaiman Sadruddin Ladhani, Head, Department of Chest Medicine, Prince Aly Khan Hospital, Aga Hall, Nesbit Road, Mazgaon, Mumbai-400010, Maharashtra, India.
E-mail: drsladhani@gmail.com
The current pandemic of Coronavirus Disease-2019 (COVID-19) has posted unprecedented challenges to the community of clinicians in various aspects, ranging from prompt and early diagnosis to preventing complications. What makes the challenge even tougher is to be able to distinguish between diseases presenting with similar complaints, especially in tropical regions, and yet be able to treat judiciously and give a targeted treatment. The level of difficulty escalates when a patient with Solid-Organ Transplant (SOT) on immunosuppressive therapy presents to the clinician as suspected COVID-19 along with a co-infection. Incidences like these carry an increased burden of higher morbidity and mortality, with or without immunosuppression, if not timely diagnosed and judiciously treated, thus heralding the need to be vigilant in the current pandemic. Thus, to the best of our knowledge, this was the first documented and successfully treated case of a patient with past history of Liver Transplant (LT) with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pneumonia with co-existing Plasmodium vivax (P.vivax) malaria.
Coronavirus disease-2019, Immunosuppressive therapy, Plasmodium vivax, Plasmodium vivax malaria, Working challenges
Case Report
A 38-year-old male with a body mass index of 29.4 kg/m2, a case of hypertension, remote smoker and a history of orthotopic LT for cryptogenic liver disease seven years ago, he was on regular oral medications with amlodipine, aspirin, tacrolimus, prednisolone and mycophenolate mofetil and past history of pulmonary tuberculosis two years back having completed anti tubercular therapy, presented to emergency medical services with acute onset febrile illness, throat irritation, loss of sense of smell, lethargy since five days. On admission, patient had a heart rate of 110 per minute, temperature 102°F, he was in mild respiratory distress, non-invasive blood pressure of 130/90 mmHg, respiratory rate of 22 breaths per minute and oxygen saturation of 93% on room air. Bilateral lung auscultation revealed normal breath sounds with unremarkable cardiovascular and other systems.
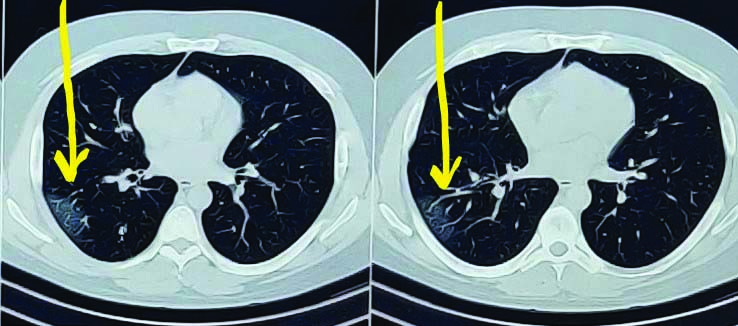
With the current pandemic situation, high clinical suspicion of COVID-19 was made. Nasopharyngeal swab for SARS CoV-2 was tested. RT-PCR for SARS CoV-2 was tested positive. Chest roentgenogram revealed no significant abnormalities. Computed Tomography (CT) of the chest revealed patchy areas of ground glass opacities noted in right lower lobe and left upper lobe, Cycle Threshold (CT) severity score 3/25 [Table/Fig-1,2]. His blood investigations revealed haemogram within normal limits, elevated serum bilirubin was 2.3 mg/dL, while other liver enzymes and coagulation profile were in the normal range. High levels of D-dimer was 5000 mg/dL, C-reactive protein was 166 mg/L, ferritin levels 1721 ng/mL, serum lactate dehydrogenase was 658 U/L and serum creatinine was 1.86 mg/dL [Table/Fig-3]. Electrocardiogram showed sinus tachycardia, arterial blood gas analysis on room air revealed mild hypoxemia.
Computed Tomography (CT) axial view of chest.
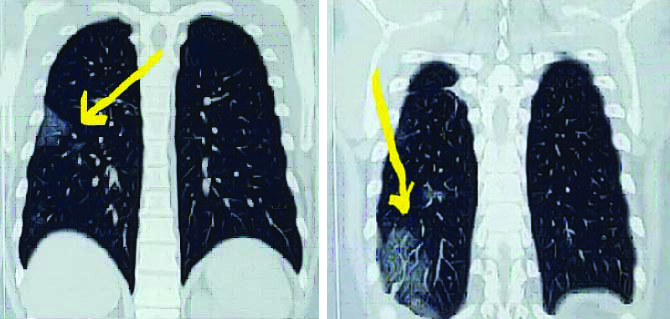
Coronal view: Computed Tomography (CT) scan of chest, arrow shows few patchy areas of ground glass opacities with interlobular septal thickening scattered in right lower lobe and left upper lobe.
Serial blood investigations and trends; peripheral smear for malarial parasites (thick and thin smear) showed ring and schizhont forms of P.vivax.
| Investigations | (Reference-units) | Days of hospital stay |
|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|
| Haemoglobin (gm/dL) | 13-17 | 15.2 | 15 | 14 | 13.5 | 14.5 |
| Total leucocyte counts (per cumm) | 4000-10000 | 6000 | 6030 | 14160 | 8530 | 8000 |
| Platelets counts (per cumm) | 150000-400000 | 159000 | 75000 | 52000 | 98000 | 118000 |
| Aspartate aminotransferase (U/L) | 0-37 | 48.8 | 59.5 | 75 | 40.1 | 32 |
| Alanine aminotransferase (U/L) | 0-40 | 50 | 53 | 55 | 45.8 | 38 |
| Total bilirubin (mg/dL) | 0-1 | 2.3 | 1.3 | 1 | 0.80 | |
| Direct bilirubin (mg/dL) | 0.1-0.3 | 1 | 0.5 | 0.5 | | |
| D-dimer (mg/dL) | 0-400 | 5000 | | 1030 | | 756 |
| C-reactive protein (mg/L) | <5 | 166 | | 184.48 | | 54 |
| Serum lactate dehydrogenase (U/L) | 230-460 | 658 | | 620 | | 500 |
| Ferritin (ng/ mL) | 30-400 | | 1721 | | | 800 |
| Serum creatinine (mg/dL) | 0.6-1.2 | 1.86 | | 1.33 | | 0.9 |
Patient was treated with supplemental oxygen via nasal prongs at four liters per minute, Intravenous (IV) ceftriaxone, dexamethasone, remdesivir, subcutaneous low molecular weight heparin, while his immunosuppressive medication tacrolimus was continued and mycophenolate mofetil was kept on hold. Bed-side ultrasonography of abdomen revealed no abnormalities. However, the patient continued to have high grade fever with chills and rigors with increasing oxygen requirement. Serial haemogram revealed falling trend in platelets counts and elevated inflammatory markers. Subsequently, on haemogram and peripheral smear examination (thick and thin smear) showed ring and schizhont forms of P.vivax which was confirmed by rapid malarial antigen test which was positive for P.vivax. Serum tacrolimus levels were within normal limits. Glucose-6-Phosphate Dehydrogenase activity (G6PD) was normal and his glycaemic control was adequate. The patient was then initiated on IV artesunate 2.4 mg/kg at 0, 12, 24 and 48 hours. He gradually improved and became symptomatically better. Subsequent serial blood investigations showed normalising platelet counts, inflammatory markers, renal and liver function tests. The patient was eventually discharged on primaquine, along with his other regular medications. He was advised home quarantine for one week and rehabilitation. On follow-up after four weeks he was clinically stable and symptom free.
Discussion
The COVID-19 has posed extraordinarily unparalleled challenges to health care workers. Towards the end of 2019, Wuhan-China, reported multiple cases of lower respiratory tract infection with clinical features similar to viral pneumonia, the cause of which was later identified as 2019 novel coronavirus (2019 nCoV) [1]. Globally, until mid February 2021, there have been 108,579,352 confirmed cases of COVID-19, including 2,396,308 deaths reported by World Health Organisation (WHO) with India having 10,916,589 confirmed cases and 155,732 deaths [2]. The clinical features of COVID-19 includes fever with or without chills and rigors, associated with cough predominantly dry, pleuritic chest pain, constitutional symptoms of bodyache, generalised fatigue and respiratory distress in severe cases while some patients may present with multisystem manifestations [1,3]. In recent times, studies have been done which bring our attention to the possibility of co-infections in COVID-19 with a variety of pathogens [3,4]. The risk of contracting COVID-19 in post SOT patients is variable with pre-existing co-morbidities and immunosuppressed status [5]. The world wide estimated data provided by WHO is 229 million cases of malaria in 2019 with an estimated death of 4,09,000 attributed to malaria [6]. Despite adoption of national programmes for prevention and control of malaria, it continues to put a burden on the socioeconomic and healthcare system [3,7]. This makes it essential to rule out the common endemic diseases, if the patient presents with symptoms overlapping in various diseases in order to prevent misdiagnosis or under-diagnosis.
Liver enzyme derangements have been very often noticed in patients of COVID-19, but the degree of derangement depends upon the severity of the disease [8]. LT patients on immune suppression therapy are at a greater risk of contracting COVID-19 but the incidence of mortality has not been widely recorded and there is very little data regarding management of LT patients with COVID-19 [8]. As reported in a study done by Fix OK et al., in post LT patient with deranged liver biochemistry, depending upon the severity of the disease it is required to continue with immunosuppressive medications in modified dosages, while off-label therapies should be continued for COVID-19 but simultaneously monitoring liver biochemistry [9]. It has been postulated in a case series report done by Patrono D et al., that increased Immunosuppression has a high tendency to delay viral clearance, thus further necessitating the need for modifying Immunosuppression therapy [10]. In a report by Bhoori S et al., 3 of 111 patients of LT with BMI >28, receiving antihypertensive drugs, with dyslipidemia and diabetes mellitus, died within a few weeks of developing SARS CoV-2 pneumonia [11] while Colmenero J et al., reported 18% mortality in LT patients with SARS-CoV-2 pneumonia requiring Intensive Care Unit (ICU) [12]. The preponderance of mortality substantially depends upon the pre-existing co-morbidities, as reported by Bhoori S et al., and Lee BT et al., [11,13].
It was observed that the patient presented with a classical presentation of COVID-19 with evidence of pulmonary involvement on CT along with blood investigations of elevated inflammatory markers, thus supporting the provisional diagnosis of SARS-CoV-2 pneumonia, the treatment for which was initiated promptly, albeit, the treatment regimen for the same is undefined and continues to be in trial. He was treated with Ribonucleic Acid (RNA) polymerase inhibitor- remdesivir since it has shown to cause inhibition of SARS-CoV-2 in human bronchial epithelial cell and because of its property of minimal interaction with tacrolimus, making it preferable over lopinavir/ritonavir [14,15]. However, regardless of appropriate treatment, he was persistently symptomatic with rapidly increasing inflammatory markers, derangements in haemogram and liver function tests, which instituted the suspicion of co infections prompting the use of combination therapy. Even though there is limited data on use of remdesivir and artesunate combination in post LT COVID-19 patients with malaria, the tolerated both under cautious monitoring.
Conclusion(s)
With the above case report, it is suggested that when a patient presents with a clinical picture which is similar in a large spectrum of diseases, clinicians should not limit their investigations and approach to the extent of current pandemic only, taking into consideration the possibilities of other tropical diseases. Also, in a patient of SOT, on immunosuppressive drugs, the need to modify immunosuppressive medicines depends upon the clinical profile of the patient. Conclusively, a holistic approach in clinical assessment, timely diagnosis along with targeted treatment, if provided to the patient can help prevent morbidity and mortality.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Feb 20, 2021
Manual Googling: Mar 18, 2021
iThenticate Software: Mar 22, 2021 (7%)
[1]. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Clinical features of patients infected with 2019 novel coronavirus in WuhanChina 2020 395(10223):497-506.10.1016/S0140-6736(20)30183-5 [Google Scholar] [CrossRef]
[2]. World Health Organisation. COVID 19 live updates. https://covid19.who.int/ [Google Scholar]
[3]. Sardar S, Sharma R, Alyamani TY, Aboukamar M, COVID-19 and Plasmodium vivax malaria co-infectionIDCases 2020 21:e0087910.1016/j.idcr.2020.e0087932665888 [Google Scholar] [CrossRef] [PubMed]
[4]. Lai CC, Wang CY, Hsueh PR, Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents?Journal of Microbiology, Immunology and Infection 2020 May 23 10.1016/j.jmii.2020.05.01332482366 [Google Scholar] [CrossRef] [PubMed]
[5]. Fishman JA, Roberts MB, Zhang EW, Kumar D, Hirsch HH, Maggiore U, Case 29-2020: A 66-year-old man with fever and shortness of breath after liver transplantationNew England Journal of Medicine 2020 383(12):1168-80.10.1056/NEJMcpc200498232937051 [Google Scholar] [CrossRef] [PubMed]
[6]. WHO. Malaria. World Health Organisation Fact Sheet. Updated 30 November 2020. Available: http://www.who.int/news-room/factsheets/detail/malaria/ [Google Scholar]
[7]. Kumar A, Valecha N, Jain T, Dash AP, Burden of malaria in India: Retrospective and prospective viewThe American Journal of Tropical Medicine and Hygiene 2007 77(6_Suppl):69-78.10.4269/ajtmh.2007.77.69 [Google Scholar] [CrossRef]
[8]. Niknam R, Malek-Hosseini SA, Hashemieh SS, Dehghani M, COVID-19 in liver transplant patients: Report of 2 cases and review of the literatureInternational Medical Case Reports Journal 2020 13:31710.2147/IMCRJ.S26591032801943 [Google Scholar] [CrossRef] [PubMed]
[9]. Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statementHepatology 2020 72(1):287-304.10.1002/hep.3128132298473 [Google Scholar] [CrossRef] [PubMed]
[10]. Patrono D, Lupo F, Canta F, Mazza E, Mirabella S, Corcione S, Outcome of COVID-19 in liver transplant recipients: A preliminary report from Northwestern ItalyTransplant Infectious Disease 2020 22(5):e1335310.1111/tid.1335332500942 [Google Scholar] [CrossRef] [PubMed]
[11]. Bhoori S, Rossi RE, Citterio D, Mazzaferro V, COVID-19 in long-term liver transplant patients: Preliminary experience from an Italian transplant centre in LombardyThe lancet Gastroenterology & Hepatology 2020 5(6):532-33.10.1016/S2468-1253(20)30116-3 [Google Scholar] [CrossRef]
[12]. Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patientsJournal of Hepatology 2020 74(1):148-55.10.1016/j.jhep.2020.07.04032750442 [Google Scholar] [CrossRef] [PubMed]
[13]. Lee BT, Perumalswami PV, Im GY, Florman S, Schiano TD, Da BL, COVID-19 in liver transplant recipients: An initial experience from the US epicenterGastroenterology 2020 159(3):1176-78.10.1053/j.gastro.2020.05.05032442561 [Google Scholar] [CrossRef] [PubMed]
[14]. Jamir I, Lohia P, Pande RK, Setia R, Singhal AK, Chaudhary A, Convalescent plasma therapy and remdesivir duo successfully salvaged an early liver transplant recipient with severe COVID-19 pneumoniaAnnals of Hepato-biliary-pancreatic Surgery 2020 24(4):52610.14701/ahbps.2020.24.4.52633234758 [Google Scholar] [CrossRef] [PubMed]
[15]. Laracy JC, Verna EC, Pereira MR, Antivirals for COVID-19 in solid organ transplant recipientsCurrent Transplantation Reports 2020 :1-1.10.1007/s40472-020-00304-z33101837 [Google Scholar] [CrossRef] [PubMed]