Breast cancer is the most common malignancy in females worldwide. To decrease mortality rates, it is crucial to detect the tumour invasion and metastasis at an early stage [1]. Interactions between tumour and stromal cells control the two protease systems that are responsible for most of the proteolysis outside the cell: the urokinase Plasminogen Activator (uPA)/uPA receptor/plasminogen network, and the MMPs [2]. MMP-2, a main member of MMP’s, is thought to be the key enzyme for metastasis of tumour with the physiological action of degrading type IV collagen.
MMP-2 is over expressed in a variety of malignant tumours and their expression and activity are often associated with tumour aggressiveness and a poor prognosis [3-5]. Therefore, MMP-2 serves as a prognostic marker in breast carcinoma regardless of patient age, disease stage, malignancy grade, or hormone receptor status and modulation of MMP-2 expression and activation provides a new mechanism for breast cancer treatment. The aim of the study was to evaluate the expression of MMP-2 in breast carcinoma tumour cells and peritumoural stroma and also to analyse the findings with the existing other prognostic markers of mammary carcinoma.
Materials and Methods
This was a retrospective study on paraffin blocks of 90 cases of invasive breast carcinoma specimens received in the Department of Pathology at Sri Ramachandra Medical College and Hospital, SRIHER, Chennai, Tamil Nadu, India, from January 2012 to June 2017 (the cases were collected from 2012 to 2017 and analysis was done in October and November, 2017). Permission of the Institutional Ethics Committee was obtained prior to commencing the study (REF: CSP-MED/16/JAN/27/12).
Inclusion criteria: Microscopically proven cases of invasive breast carcinoma of all histological types. Mastectomy and wide local resection specimens received during the period of January 2012 to June 2017.
Exclusion criteria: Benign breast lesions. Breast malignancies other than carcinomas. Incision biopsy specimens without mastectomy.
The clinical data of patients including age, gender and stage were obtained from the medical records section. The histopathological data were collected from the pathological case files. Paraffin blocks which contained the tumour with adjacent tissue were collected for the study. Five micron sections were cut and stained with Haematoxylin and Eosin (H&E). Tumours were type specified and stage based on World Health Organisation (WHO) guidelines 2019 [6].
Invasive breast carcinomas were graded based on the Nottingham combined histologic grade [Table/Fig-1] (Elston-Ellis modification of Scarf-Bloom-Richardson grading system) [7,8].
a: Modified Radical Mastectomy specimen showing Infiltrating mammary carcinoma with lymphnode metastasis; b: Infiltrating Ductal carcinoma No Special Type (NST) (arrow) (H&E 200x); c: Infiltrating lobular carcinoma (H&E 200X); d: Infiltrating ductolobular carcinoma (H&E 200X).

Immunostaining for ER was done using monoclonal antibody to ER, prediluted antibody, procured from BiogenexLaboratories. Immunostaining for PR was done using mouse monoclonal antibody to PR (Clone: PR88), procured from BiogenexLaboratories. Immunostaining for HER2 was done using monoclonal antibody to c-erbB-2 Protein (HER2 Prediluted Antibody, procured from Biogenex Laboratories. ER/PR and HER2 staining were reported as per the American Joint Committee on Cancer Protocol guidelines for molecular subtyping. A cut-off of a minimum of 1% of tumour cells showing nuclear positivity for ER/PR was considered positive (American Society of Clinical Oncologist ASCO guidelines, 2010) [9].
The cases were classified according to the molecular classification based on the ER, PR and HER2 receptor status [Table/Fig-2].
Molecular classification.
| Molecular subtypes | ER | PR | HER2 |
|---|
| Luminal A | + | + | - |
| Luminal B | + | + | + |
| HER2neu | - | - | + |
| Triple negative | - | - | - |
ER: Oestrogen receptor; PR: Progesterone receptor; HER: Human epidermal growth factor receptor 2
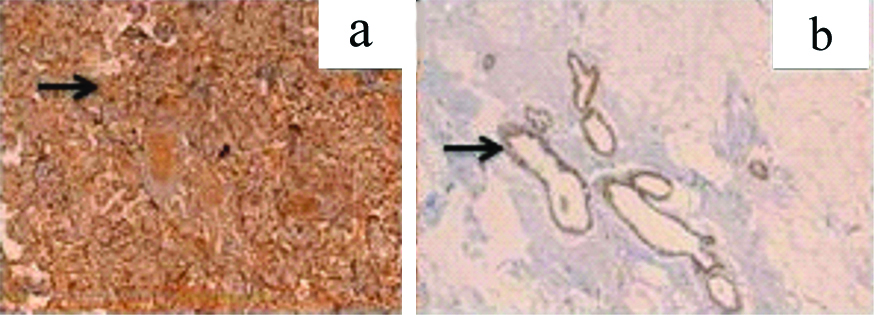
Immunohistochemical staining for MMP-2 was done using IgG (Immunoglobulin G) Rabbit polyclonal antibody with immunogen range 20-70/476, Bioss, USA. MMP-2 is a cytoplasmic marker. Human placental tissue was taken as positive control-cytotrophoblasts, syntitrophoblasts as well as blood vessels were stained positive for MMP-2. Normal breast tissue was also stained positive for MMP-2 which served as an inbuilt control [Table/Fig-3].
a: Positive control of MMP-2 cytoplasmic staining (arrow) in normal placenta-IHC 200X; b: MMP-2 immunostain in normal ductal epithelium IHC-100X.
Assessment of MMP-2
The scoring system used in this study is Immunoreactive Score (IRS) scoring system for cytoplasmic staining in the tumour cells [Table/Fig-4] [10].
Immunoreactive Score (IRS) [10].
| Percentage of tumour cells | Intensity of staining | IRS score |
|---|
| 0-no positive cells | 0-negative | 0-1=negative |
| 1-<10% positive cells | 1-mild | 2-3=mild |
| 2-10-50% positive cells | 2-moderate | 4-8=moderate |
| 3-51-80% positive cells | 3-intense | 9-12=strongly positive |
| 4->80% positive cells | | |
The total score was calculated from the sum of the staining intensity and extent of tumour cells positive. The scoring system used by Catteau X et al., for staining of stromal MMP-2 was also used in this study [11]. A two grade system was used to score the stromal expression of MMP-2 with a cut-off of 10% which was classified as positive or negative.
Statistical Analysis
Statistical analysis was done on the data collected by using the software GNU-PSPP version 0.10.1. Pearson Chi-square test was used to determine significant clinicopathological differences between MMP-2 expression in positive and negative tumours. Differences were considered statistically significant when p-value was <0.05.
Results
The study included 90 cases of histological proven invasive breast carcinoma. The study parameters include age, laterality, size of the tumour, clinical staging, histopathological grade, lymphnode status, molecular subtype and MMP-2 expression in invasive breast carcinoma [Table/Fig-5,6 and 7].
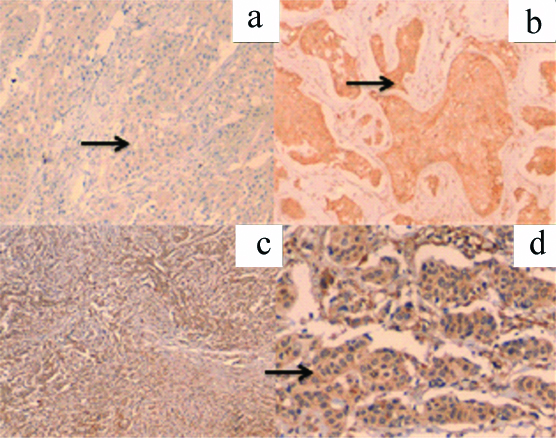
a: MMP-2 positive cytoplasmic staining (arrow)-1+ (IHC-200X); b: MMP-2 positive staining (arrow)-2+ (IHC-200x); c: MMP-2 positive staining -3+ (IHC-100X) d: MMP-2 positive staining(arrow)-3+ (IHC-200X)
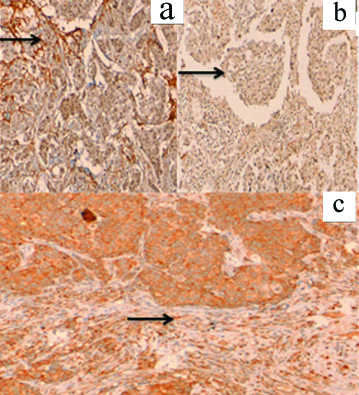
a: Positive 3+ cytoplasmic expression (arrow) of tumour and stromal MMP-2 -IHC 200x; b:Positive 1+ expression (arrow) of tumour and stromal MMP-2 -IHC 200x; c: Positive 2+ expression (arrow) of tumour and stromal MMP-2 -IHC 200x.
MMP-2 expression in tumour cells as related to clinicopathological parameters.
| Parameters | MMP-2 positive | MMP-2 negative | Total no. of cases | p-value* |
|---|
| Age (years) |
| 21-40 | 6 | 9 | 90 | 0.869 |
| 41-60 | 28 | 20 |
| >60 | 18 | 9 |
| Tumour stage |
| T1 | 7 | 4 | 90 | 0.031 |
| T2 | 33 | 22 |
| T3 | 12 | 5 |
| T4 | 0 | 7 |
| Nodal status |
| Node positive | 25 | 10 | 90 | 0.382 |
| Node negative | 27 | 28 |
| Tumour grade |
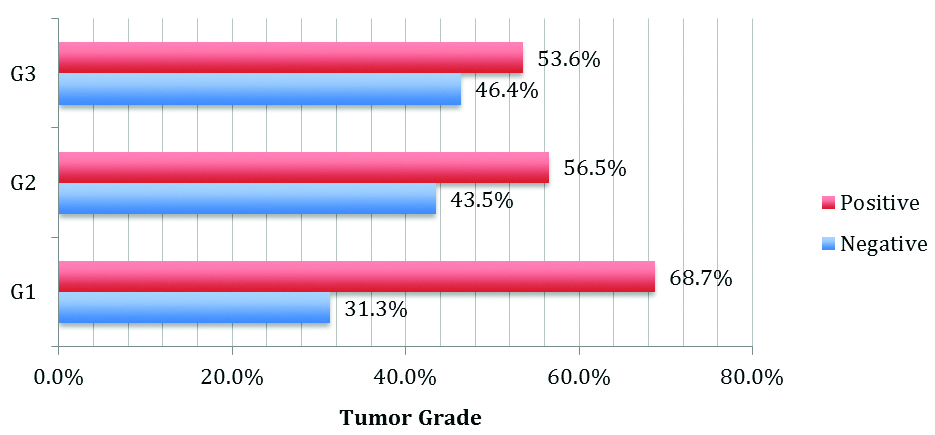
| 1 | 11 | 5 | 90 | 0.748 |
| 2 | 26 | 20 |
| 3 | 15 | 13 |
| HGDCIS |
| Present | 26 | 15 | 90 | 0.020 |
| Absent | 26 | 23 |
| ER |
| Positive | 34 | 20 | 90 | 0.292 |
| Negative | 18 | 18 |
| PR |
| Positive | 25 | 16 | 90 | 0.573 |
| Negative | 27 | 22 |
| HER2 |
| Positive | 9 | 13 | 90 | 0.059 |
| Negative | 43 | 25 |
*p-value <0.05 considered as significant, tested by using the Pearson Chi-square test
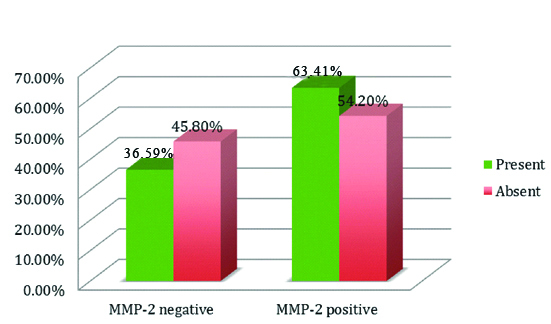
Out of 90 cases, 52 cases were positive for MMP-2 and 38 cases were negative for MMP-2 in tumoural cells. In the peritumoural stroma 18 cases were positive and 72 cases were negative surrounding the peritumoural stroma.
Expression of MMP-2 in Tumour Cells
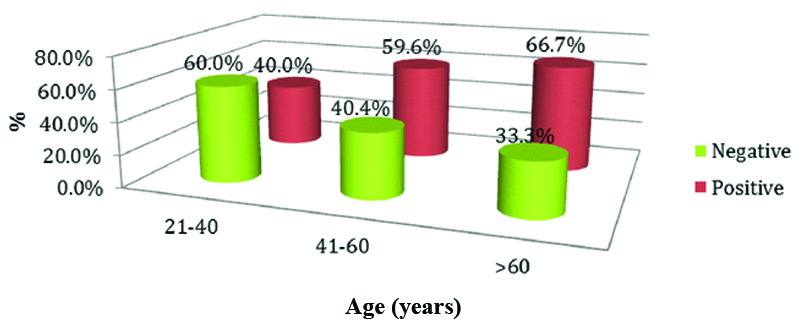
Increased expression of MMP-2 was observed in the age group of >60 years which was statistically insignificant (p-value=0.869) by using the Pearson Chi-square test [Table/Fig-8].
Expression of MMP-2 in various age groups.
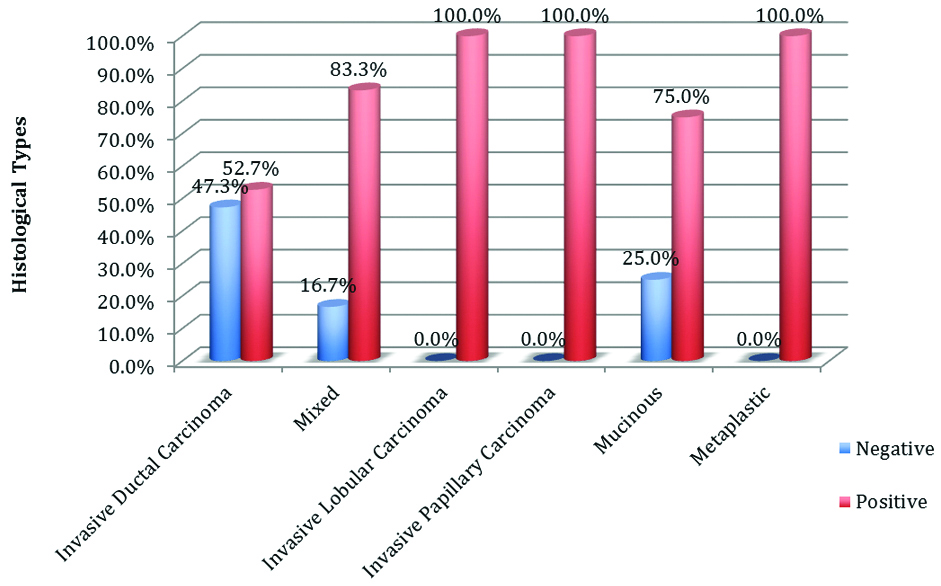
A total of 39 cases of Infiltrating Ductal Carcinoma (IDC), No Special Type (NST) showed positive expression for MMP-2. Statistically insignificant association was seen (p-value=0.776) by using the Pearson Chi-square test [Table/Fig-9].
Expression of MMP-2 in various histological types.
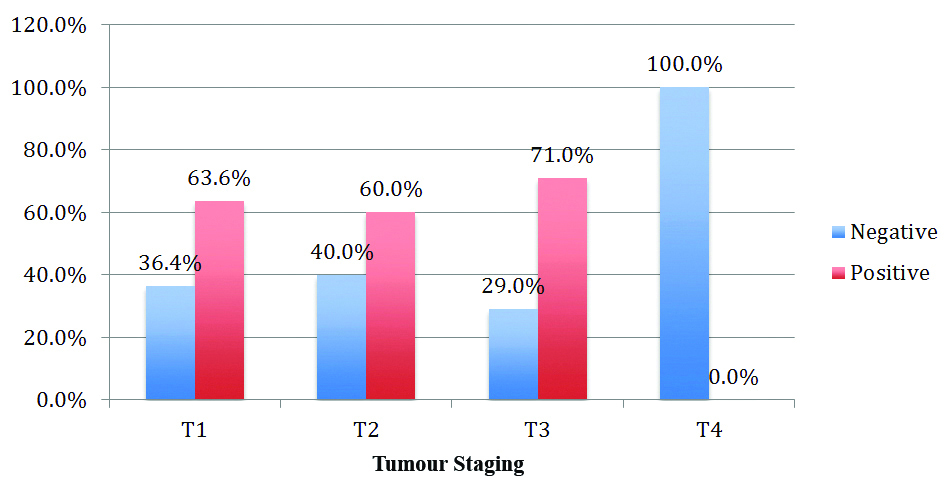
Expression of MMP-2 with tumour stage appeared statistically significant with p-value=0.031, tested by using the Pearson Chi-square test [Table/Fig-10].
Expression of MMP-2 and tumour stage.
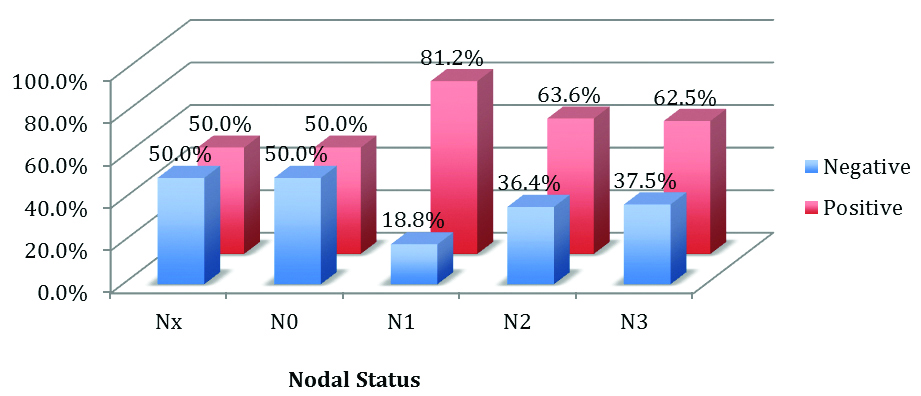
Statistically insignificant association was seen between MMP-2 and Nodal status (p-value=0.382) by using the Pearson Chi-square test [Table/Fig-11].
Expression of MMP-2 and nodal status.
Out of 41 cases of high grade DCIS, 26 cases showed positive staining for MMP-2 with statistically significant association (p-value=0.020) tested by using the Pearson Chi-square test [Table/Fig-12].
Association of MMP-2 with ductal carcinoma in situ.
The association between MMP-2 immunostaining and tumour grade (p-value=0.748) was tested by using the Pearson Chi-square test [Table/Fig-13].
Expression of MMP-2 in various tumour grade.
Expression of MMP-2 in Stroma
MMP-2 staining was also expressed in the stroma surrounding the tumour cells. Its expression was also studied in the 90 cases of invasive breast carcinoma and compared with the clinicopathological parameters. Insignificant association was seen with ER, PR status and HER2 neu expression of MMP-2 with p-value of 0.218, 0.313 and 0.284 respectively. No significant association between expression of MMP-2 in the peritumoural stroma and age, Grade, tumour stage, nodal status and hormonal receptor status (p-value >0.05) [Table/Fig-14].
MMP-2 expression in stromal cells as related to clinicopathological parameters.
| Parameters | Stromal MMP-2 positive | Stromal MMP-2 negative | Total no of cases | p-value* |
|---|
| Age |
| <50 y | 8 | 26 | 90 | 0.348 |
| >50 y | 10 | 46 |
| Tumour stage |
| T1 | 2 | 9 | 90 | 0.390 |
| T2 | 11 | 45 |
| T3 | 5 | 11 |
| T4 | 0 | 7 |
| Nodal status |
| Node positive | 7 | 28 | 90 | 0.506 |
| Node negative | 11 | 44 |
| Tumour grade |
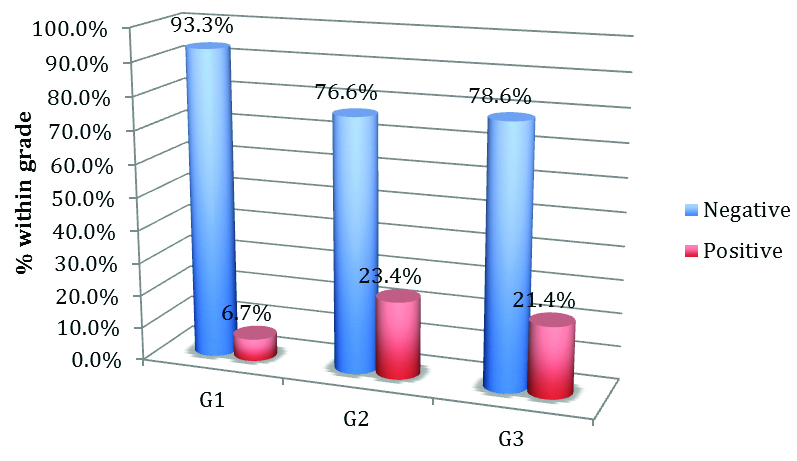
| 1 | 1 | 14 | 90 | 0.346 |
| 2 | 11 | 36 |
| 3 | 6 | 22 |
| HGDCIS |
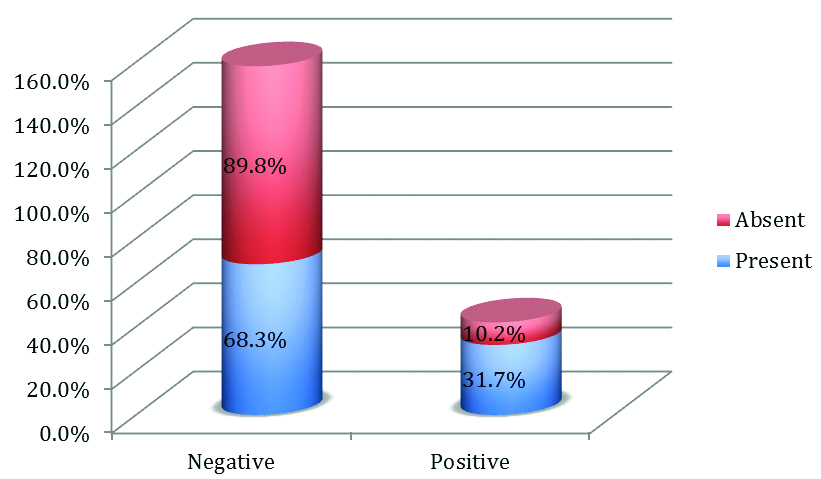
| Present | 13 | 28 | 90 | 0.013 |
| Absent | 5 | 44 |
| ER |
| Positive | 14 | 40 | 90 | 0.218 |
| Negative | 4 | 32 |
| PR |
| Positive | 11 | 30 | 90 | 0.313 |
| Negative | 7 | 42 |
| HER2 |
| Positive | 2 | 20 | 90 | 0.284 |
| Negative | 16 | 52 |
*p-value <0.05 considered as significant tested by using the Pearson Chi-square test
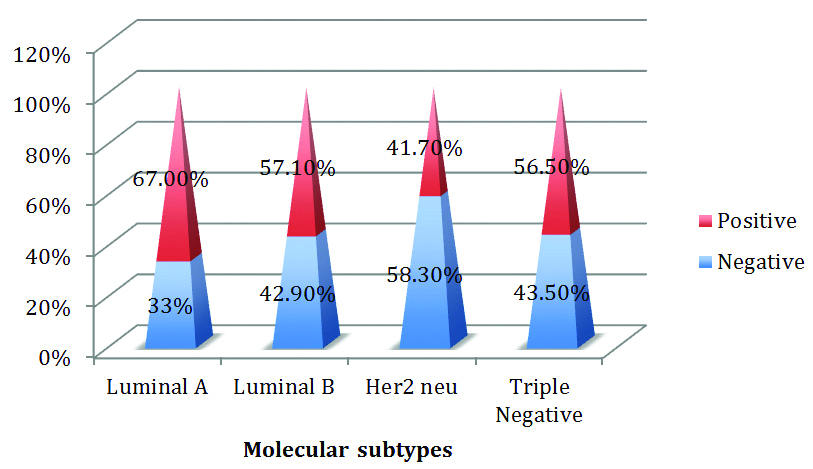
Among the molecular subtypes, 26 (67%) luminal type A, 8 (57.1%) luminal type B and 5 (41.70%) of HER2, 13 (56.5%) of the 23 triple negative cases were positive for MMP-2. The higher proportion of MMP-2 positive tumours fell in the Luminal A category [Table/Fig-15].
Expression of MMP-2 among various molecular subtypes.
Increased expression of stromal MMP-2 seen in the grade 2 tumours (p-value=0.346) was tested by using the Pearson Chi-square test [Table/Fig-16].
Expression of stromal MMP-2 in tumour grade.
Significant association was noted in stromal MMP2 expression in high grade DCIS, 41 tumours had high grade DCIS in which 13 cases showed positive staining for stromal MMP-2. (p-value=0.013) tested by using the Pearson Chi-square test [Table/Fig-17].
Expression of Stromal MMP-2 in Ductal Carcinoma In situ.
Discussion
The various prognostic factors that determine patient therapy and outcome include age, tumour burden, histological type, grade, lymphnode status and hormone receptor status.
MMP-2 contributes a major role in cell migration by degrading the extracellular matrix component. MMP-2 enzyme is responsible for both physiological and pathological processes. A study by Jezierska A and Motyl T, found an association of MMP-2 protein in breast carcinoma tumour cells with decreased recurrence free survival rate [12].
MMP-2 is an enzyme that degrades components of the extracellular matrix and thus plays a pivotal role in cell migration during physiological and pathological processes such as gastric, pancreatic, prostate and breast cancer. MMP-2 protein is found in breast carcinoma tumour cells is associated with shortened recurrence free survival or relative overall survival [12].
MMP-2 expression was noted in 70% of cases in the tumour cells and 20% of cases in the peritumoural stroma. This finding was in concordance with a study done by Nakopoulou L et al., and Emonard HP et al., which claim that the existence of an MMP-2 binding site on the tumoural cell membranes is responsible for host fibroblast secreted enzyme [13,14]. In this study MMP-2 was expressed in Stage III as well as Stage I breast cancer cells. The high expression of MMP-2 was observed in 71% of cases in Stage III cancer cells and 63% of cases in Stage I cancer cells. These findings correlated well with statistically significant p-value=0.031. These findings were in concordance with the study done by Pellikainen JM et al., and Mahmood NA where they observed increased expression of MMP-2 in stage III as well as stage I disease [15,16].
Thus tumour stage depends on the tumour size where the MMP2 activity increases as the tumour size increases this finding was observed in a study done by Rha SY et al., [17]. This was observed in the present study as well.
Association of MMP-2 in the peritumoural stroma with the tumour stage was statistically insignificant with a p-value of 0.39. This finding was in concordance with the study done by Nakopoulou L et al., [13]. MMP-2 stromal expression was increased in higher stage where the tumour size is more >2 cm. This was observed in the studies done by Catteau X et al., and Ranogajec I et al., [11,18].
In present study, MMP-2 was highly expressed in both tumoural cells and stroma of high grade DCIS which was statistically significant with a p-value of 0.02 and 0.01, respectively. Gonzalez LO et al., showed that high expression of MMP-2 by the tumour cells of the neoplastic ducts as well as by the stromal cells stating that the MMPs are implicated in tumour invasion as well as metastasis [19].
Axillary lymphnode status is the most basic prognostic factor in patients with breast cancer. Though high expression of MMP-2 is observed in node positive cases, in the present study immunostaining for MMP-2 both in the tumour as well as in the stroma was statistically insignificant with a p-value of 0.38 and 0.50, respectively. This finding was in concordance with the study done by Talvensaari-Mattila A et al., [3]. Tumour grade and MMP-2 expression did not correlate in present study. High grade tumours showed increased expression of MMP-2 in this study. Similar findings were seen in a study done by Sullu Y et al., [20]. Ramos EA et al., showed no association between MMP-2 and histological types, a similar finding seen in this study [21].
In the current study, higher expression of MMP-2 was observed in the age group of >50 years, there was no significant association of MMP-2 expression both in the tumoural cells as well as in the stroma which was statistically insignificant with a p-value of 0.86 and 0.34, respectively. This was observed in the study done by Kim G et al., [22].
ER and MMP-2 have shown a molecular relationship with E2 or estradiol binding in the E domains of ER, present in the plasma membrane. This interaction could activate MMP-2 with subsequent transactivation of Endothelial Growth Factor Receptor (EGFR). This mechanism after ER and MMP-2 interaction supports the proliferation and survival of tumour cells in the absence of nuclear ER.
No association was found between the molecular subtypes and MMP-2 protein, which was statistically insignificant (p>0.67) as seen in the study done by Talvensaari-Mattila A et al., [3]. On the contrary, a study done by Abbas NF et al., found high association with ER and PR status (p=0.034) [23].
In the present study, high expression of MMP-2 was seen Luminal A subtype followed by triple negative and Luminal B subtypes. This study was contrary to the study done by Sullu Y et al., where he found increased expression of MMP-2 in Triple Negative tumours and low expression was seen in Luminal A tumours [20].
In the present study, there was no significant association of MMP-2 with ER, PR and HER2 (p>0.05). In view of the above findings it can be stated that MMP-2 expression can be analysed as a prognostic marker in mammary carcinoma.
Limitation(s)
The sample size was small due to logistical reasons and patient survival data was not analysed. Expression of MMP-2 with distant metastasis was also not studied since all the cases in present study were cM0 (clinically no distant metastasis). Thus the future scope includes increasing the sample size and including cases with metastasis and correlating with the survival data of the patients.
Conclusion(s)
From present study it can be concluded that there was high MMP-2 immunohistochemical expression in breast carcinoma. There was statistically significant association of increased MMP-2 with tumour stage. There was also statistically significant association of MMP-2 in the tumour and stroma with high grade DCIS. There was increased expression of MMP-2 in Luminal A subtype. Low expression was seen in the other molecular subtypes. In view of these findings and association with other studies in the literature, the present study demonstrates that the expression of MMP-2 in tumour and stromal cells could serve as a parameter of poor prognosis in breast cancer.
ER: Oestrogen receptor; PR: Progesterone receptor; HER: Human epidermal growth factor receptor 2
*p-value <0.05 considered as significant, tested by using the Pearson Chi-square test