The Chronic Urticaria (CU) is a distressing problem and patients of CU suffer from the morbidity that arises from irritable itch and wheals. They are also subjected to a huge antihistamine pill burden. CU is a distressing dermatosis of skin and is defined as urticaria persisting daily or almost daily for more than six weeks and where a predominant physical cause has been excluded. Approximately, 20% of the general population can have a history of urticaria once in their lifetime [1]. Prevalence of CU in world population can vary from 0.1 to 1% [2]. CU can be associated with the various auto-immune condition including thyroid disorder, vitiligo, rheumatoid arthritis and pernicious anaemia. It is also associated with a significant effect on the quality of life of the patient.
Currently, antihistamines which generally work by alleviating the symptoms rather than enacting a cure, are the standard treatment for CU. Modern second generation antihistamines has always been considered as the first line symptomatic treatment for urticaria because of their overall good safety profile, proven efficacy, and a broad spectrum of controlling urticarial/angioedema pathological cascades, followed by a later escalation of the dosage of these agents and adding a leukotriene antagonist as the next strategies in the management. The use of immunomodulators such as Ciclosporin, methotrexate, omalizumab, and dapsone is the last resort in treating this condition. CU has an unpredictable, relentless course and often shows a poor response to drug treatment. It can be challenging to determine the appropriate medication to suit the patient. The need for long term and daily intake of these agents is frustrating and may lead to non-compliance. Treatment options which increase the duration of remission and those which address the key factors involved in the disease pathogenesis are the need of the hour.
Thus, the present study was done to assess the effectiveness of injection histaglobulin, a complex of histamine and human immunoglobulin, in producing relief in patients with CU. Authors also compared response to injection histaglobulin in ASST positive and ASST negative group of CU patient.
Materials and Methods
A single centre, open label, non-randomised prospective clinical study was carried out in the Dermatology Venereology and Leprosy (DVL) Out Patient Department (OPD) in our institute from April 2016 to July 2017 after obtaining the approval from the Institutional Ethics Committee.
Inclusion criteria: Patients diagnosed as chronic spontaneous urticaria of either gender presenting to OPD were included in study after obtaining informed consent. Chronic spontaneous urticaria was diagnosed if the patient had a history of daily or almost daily occurrence of widespread, itchy, spontaneous wheals for a period of six weeks or more, with individual lesions lasting less than 24 hours after excluding physical causes.
Exclusion criteria: Patients younger than 18 years, pregnant and lactating mothers, patients on long term immunosuppressants for CU and those with any other co-morbidity were excluded from the study. Physical urticaria such as cold induced, pressure, heat contact, solar, dermographic, vibratory, aquagenic, cholinergic, and drug induced urticaria were also excluded from the study.
Sample size: 40 patients of chronic spontaneous urticaria presenting to outpatient department were enrolled in the study after excluding physical urticaria.
Baseline Evaluation
After obtaining informed consent baseline evaluation was done. This comprised a detailed history regarding the duration, severity, diurnal variation of lesions, any systemic co-morbidities, such as thyroid disorders, diabetes mellitus, or hypertension, any concomitant drug use by the patient and a history suggestive of any physical form of urticaria or urticarial vasculitis. Female patient’s status on pregnancy or lactation was also noted. This was followed by a clinical examination of the patient. The baseline UAS 7 score was calculated, and the number and type of oral antihistamine the patient was taking in the past week were recorded. The UAS 7 was calculated based on European Academy of Allergy and Clinical Immunology (EAACI) guideline 2013 [3]. UAS 7 a unified, validated and simple scoring system. It is a sum of daily pruritus score (0-none, 1-mild, 2-moderate, 3-severe) and daily wheal score (0-none, 1-one to six wheals, 2-seven to twelve wheals, 3-more than 12 wheals). The sum of the score (0-6) for each day is summarised over one week, with a maximum of 42. Higher scores indicate more severe disease.
Routine investigations such as complete blood count with differential count, urine routine examination, stool examination for ova and cysts, thyroid profile, and renal and liver function tests, were performed. ASST was then performed as per the standard procedure [4]. Accordingly, patients were divided into subgroups as ASST ‘positive’ patients and ASST ‘negative’ patients.
Treatment Schedule and Follow-up
Histaglobulin: The patients were administered a subcutaneous injection of 1 mL Histaglobulin (HISTOGLOB® manufactured by Bharat Serums and Vaccines Ltd., Maharashtra, India), which is a combination of human normal immunoglobulin (12 mg) and histamine dihydrochloride (0.15 mcg). The recruited patients were administered 1 mL of histaglobulin subcutaneously over the arm, weekly for eight consecutive weeks. Patients were also prescribed rescue medication (tablet levocetirizine 5 mg) to be taken when required, not exceeding the permitted daily dosage. During each weekly visit, the response to treatment was assessed using UAS 7. The number of rescue medications taken in the past week was also noted. A final assessment was done at 24th week of starting the therapy.
Statistical Analysis
Statistical analysis was performed using Statistical Analysis System (SAS®), Version 9.4. Descriptive statistics such as mean, standard deviation, median, minimum and maximum were calculated for continuous variables, while frequency and percentage were calculated for categorical variables. Analysis of Variance (ANOVA) with post-hoc test was used to compare baseline, week 8 and week 24 UAS 7 scores. Independent t-test and unpaired t-test were applied to compare mean UAS 7 score between gender and mean UAS 7 score in ASST ‘positive’ and ASST ‘negative’ groups, respectively.
Results
Study Population
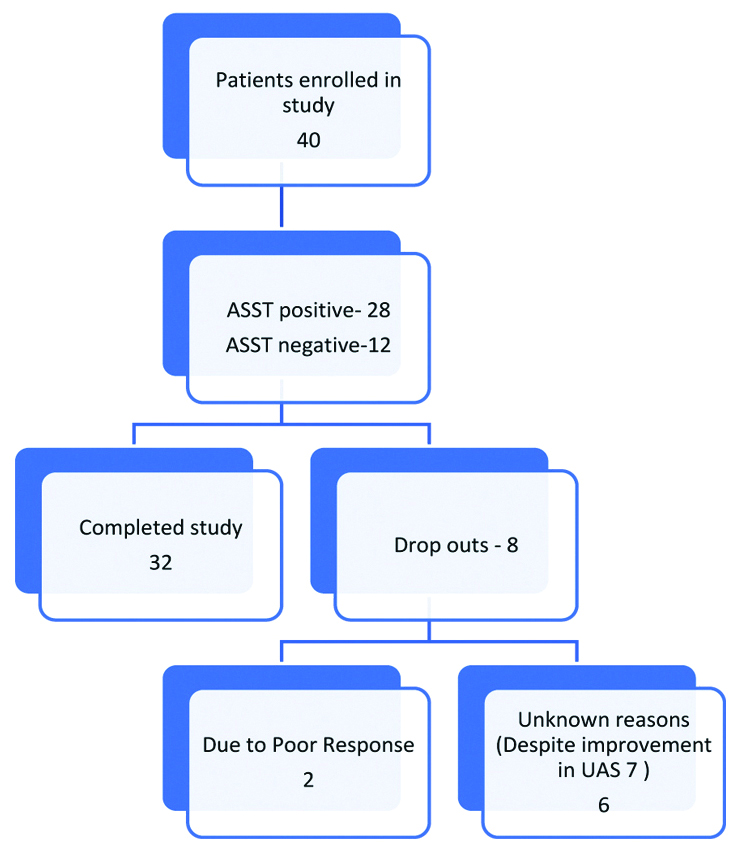
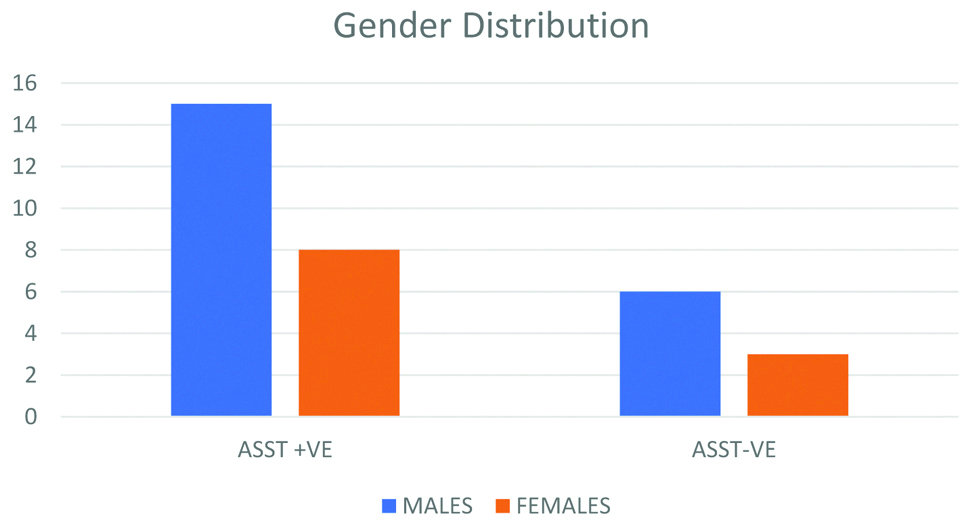
Forty patients were included in the study. Of them, 32 patients completed the stipulated eight weeks of treatment. Eight out of 40 patients dropped out from the study in the course of the study, out of which two had a poor response to treatment and other six opted out due to unknown reasons even after improvement in UAS 7 score [Table/Fig-1]. Amongst 40 patients enrolled, 26 were males and 14 were females. The mean age of the patients was 32.8 years. Mean duration of the disease was 18 weeks. ASST was negative in 9 patients (28%) and positive in 23 patients (72%) who completed the study [Table/Fig-2].
Assessment of UAS 7 Score at Baseline, Week 8 and Week 24 for Male and Female Patients
Baseline UAS 7 scoring in male and female showed insignificant difference (p>0.05). It reduced to (2.57±2.23) in males and (3.57±1.86) in females at 8th week, whereas at week 24 UAS 7 values were (2.8±2.02) in males and (2.78±2.22) in females. There was no significant difference between UAS 7 scoring based on gender at week 8 and week 24 as well [Table/Fig-3].
UAS 7 score in male and female.
| Gender | Baseline | Week 8 | Week 24 |
|---|
| Male | 12.60±3.96 | 2.57±2.23 | 2.8±2.02 |
| Female | 12.78±4.02 | 3.57±1.86 | 2.78±2.22 |
| p-value** | 0.84 (NS) | 0.12 (NS) | 0.92 (NS) |
**Independent t-test was applied to compare mean UAS 7 score between gender; p-value <0.05 considered significant
Assessment of UAS 7 Score at Baseline, Week 8 and Week 24 for ASST Positive and Negative Cases
Mean UAS 7 value at baseline for ASST Positive and ASST negative group showed no significant difference. At week 8 ASST positive and ASST negative group also had no significant difference but at week 24 it showed statistically significant difference {3.21±2.21 vs 1.83±0.93 (p<0.05)} [Table/Fig-4].
UAS 7 score in ASST positive and ASST negative patients.
| ASST | Baseline | Week 8 | Week 24 |
|---|
| ASST Positive (23) | 13.28±3.95 | 3±2.26 | 3.21+2.21 |
| ASST Negative (9) | 11±3.54 | 1.58±1.83 | 1.83±0.93 |
| p-value** | 0.09 (NS) | 0.06 (NS) | 0.04 (S) |
**Unpaired t-test was applied to compare mean UAS 7 score between ASST Positive and ASST negative groups; p-value <0.05 considered significant
Efficacy
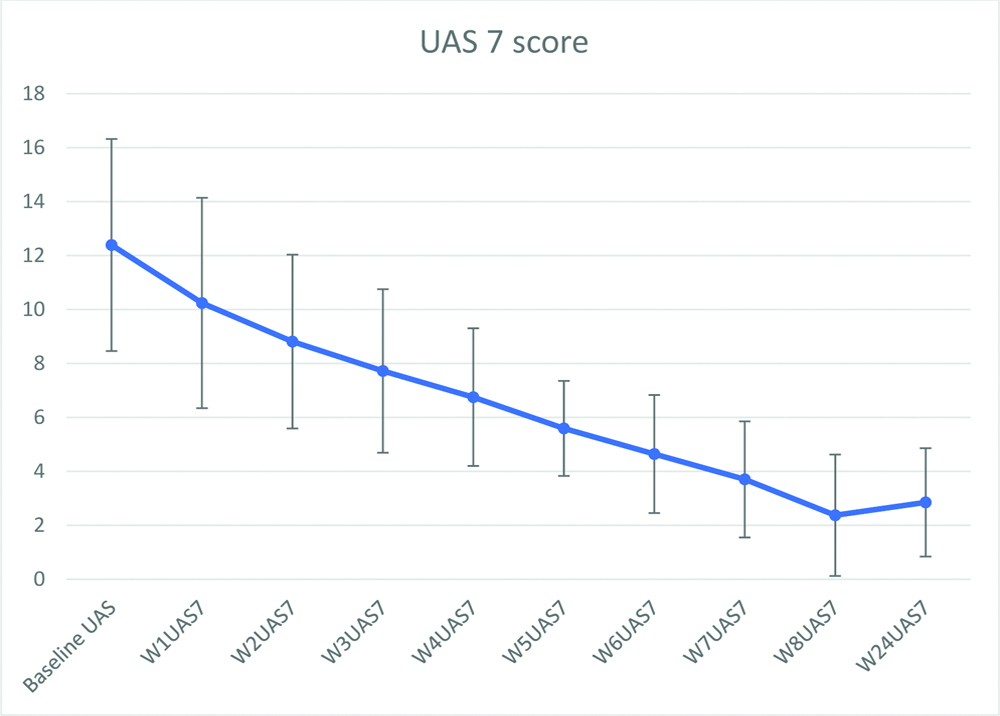
Mean UAS 7 value reduced significantly by 8 weeks (p<0.05) and mean UAS 7 value at 24 weeks was (2.8±2.01) [Table/Fig-5]. Weekly reduction in UAS 7 score is shown in [Table/Fig-6]. Mean number of antihistamines taken per week by patients at baseline evaluation was 8.12 and which was statistically reduced post-treatment (1.71, p<0.05). Out of 32 patients who completed study 21 patients did not require to continue antihistamine. Rest of all 11 patients who completed the study also showed a reduction in antihistamine requirement [Table/Fig-7].
UAS 7 sore at baseline, week 8 and Week 24.
| UAS 7 score | Baseline | Week 8 | Week 24 | p-value** |
|---|
| Mean (N-32) | 12.6 | 2.57 | 2.8 | <0.0001 |
| SD | 3.93 | 2.21 | 2.01 |
**ANOVA with post-hoc test was applied; p-value <0.05 considered significant
Weekly reduction in Urticaria Activity Score (UAS) during the study period.
| No of antihistamine tab taken per week | Baseline | Week 24 |
|---|
| Mean | 8.12 | 1.71 |
| SD | 2.45 | 2.41 |
None of the patients reported any adverse effect either during or after the treatment.
Discussion
Chronic Urticaria (CU) is heterogeneous in its course, clinical picture and underlying causes. Common causes of CU include autoreactivity, physical urticaria and infection. Treatment of CU should be aimed at curing the condition whenever possible. This requires the identification of the underlying cause and measures taken against it. A 2nd generation H1 antihistamines remains first line management of CU. Their dose can be increased up to four-fold if no response is seen. Oral steroids can be used for control of severe exacerbation of CU but cannot be continued for longer duration due to side-effect profile. Omalizumab (anti-IgE) has been shown to be very effective and safe in the treatment of CU. Recommended dose of omalizumab is 300 mg once a month. Cost of therapy is concerning factor for Indian scenario. Ciclosporin A is off label drug used primarily for patients with severe disease refractory to any dose of antihistamine. Ciclosporin A is not recommended as standard treatment of CU because of variety of side-effects like hypertension and renal toxicity. There is no enough evidence to recommend therapy like methotrexate, plasmapheresis, intravenous immunoglobulins and phototherapy for standard treatment of CU even though few case series mentioned their usefulness [5].
Autologous serum therapy, autologous whole blood therapy and allergen specific immunotherapy are various immunotherapies which are been used in the management of urticaria with some success. Allergen specific immunotherapy can be used in the small number of cases of CU in which IgE mediated allergy is a causative factor [6]. Autologous whole blood therapy is one of the oldest therapies used in CU patients, which is more effective in ASST positive patients [7]. Mechanism of action of such immunotherapies is proposed to be the generation of anti-idiotype antibodies to various mast cell degranulating agents.
Histaglobulin is a sterile preparation of histamine dihydrochloride and gamma globulin derived from human blood in fixed proportion (Human Normal Immunoglobulin 12 mg, Histamine Dihydrochloride 0.15 mcg). Histaglobulin when injected into the body stimulates the production of antihistaminic antibody which fixes and neutralises the released histamine to the allergic reaction. Repeated doses of histaglobulin increase the antibody titer, for which it is recommended to administer doses every six months to maintain optimal titre of antibody [6]. Serum histamine binding capacity in a normal individual is 20-30% and in allergic patients, it is only 0-5%. Histaglobulin treatment decreases IgE levels and it is hypothesised that the serum binding capacity of a patient to histamine increases [8]. Histaglobulin has been widely used in the treatment of allergic rhinitis. It is also used in various allergic disorders such as asthma, atopic dermatitis, CU, erythema multiforme and cutaneous drug allergy [9,10]. Rudzki E and Czubalski K, first demonstrated the usefulness of histaglobulin in CU along with prurigo [11]. Gushchin IS et al., also showed the effectiveness of histaglobulin in chronic recurrent (idiopathic) urticaria along with allergic rhinitis [12].
In present study, male patients outnumbered female patient (26 vs 14) despite CU is more common in females [13]. The reason for that can be a small sample size of this study or tertiary care more accessible to male as compared to females. Mean duration of urticaria was 18 weeks. There was a significant reduction (81%) in UAS 7 from baseline to week 8 (12.6±3.93 to 2.57±2.21) (p<0.0001). Reduction in UAS 7 was well maintained at week 28 follow-up with an only marginal increase in mean UAS 7 (2.57±2.21 to 2.8±2.01) which indicates therapeutic benefit of histaglobulin maintained even after discontinuing it. Possible mechanism for this effect can be persistent level of antihistamine antibody titre even after stopping histaglobulin therapy. Prevention of upper respiratory infection in patient of atopic dermatitis was noted even after stopping histaglobulin therapy in one study [9]. It is another example of maintained histaglobulin effect after stopping the therapy. All the 32 patients who completed the study showed improvement in UAS 7. Total of 30% patients (12 patients) showed complete response at week 8 (UAS 7=Zero). Percentage of patients showing complete response was lower than the study by Godse K et al., (35.15%), Rajesh G et al., (45%) and Thota S (46.2%) [Table/Fig-8] [14-16]. Both ASST positive and ASST negative group showed a significant reduction in UAS 7 scores from baseline to week 8 which indicate that histaglobulin can be beneficial in patients of ASST negative group as well, but this finding must be validated in large prospective studies. Contrary to known fact, ASST positive and ASST negative group of patients didn’t show any significant difference between UAS 7 score at baseline and week 8, but UAS 7 score of ASST negative group at week 28 was significantly less than that of ASST positive group. This finding may be present due to the small sample size of the study. In all the patients who completed the study, antihistamine use could be reduced or stopped which proves the therapeutic benefit of histaglobulin. Thus, histaglobulin can be a useful addition in dermatologist armament against urticaria.
Comparison with other studies [14-16].
| Sr. No. | Study | Total patients enrolled N | Male/Female | ASST Positive/ASST -Ve | Mean UAS at baseline | Mean UAS at end of study | % reduction in UAS | % patient showing 100% response |
|---|
| 1 | Godse K et al., [14] | 38 | 25/13 | - | 15.8±6.06 (UAS 7) | 6.0±6.22 | | 35.15% |
| 2 | Rajesh G et al., [15] | 51 | 14/37 | 29/22 | 18.9±6.3 (UAS 7) | 3.7±5.7 | 80% | 45% |
| 3 | Thota S [16] | 35 | 10/25 | 20/15 | 5.1 | 1.01 | 80% | 46.2% |
| 4 | Present study | 40 | 26/14 | 28/12 | 12.6±3.93 | 2.57±2.21 | 81% | 30% |
Limitation(s)
The small sample size was present study’s main limitation. Absence of control group for comparing the efficacy of histaglobulin was another important limitation.
Conclusion(s)
Histaglobulin is an important adjuvant in management in CU. It can be useful in treatment in CU irrespective of ASST status. It can help to reduce the antihistamine requirement in the majority of patients. it is safe, well-tolerated effective management option other than options currently available.