Comparative Evaluation of Efficacy between Topical Calcipotriol used along with Topical Clobetasol and Topical Clobetasol Monotherapy in Treatment of Alopecia Areata: A Randomised Clinical Trial
Mohan Lal Gupta1, Shivangna Singh2, Bushra Hasan Khan3
1 Associate Professor, Department of Dermatology, FH Medical College and Hospital, Agra, Uttar Pradesh, India.
2 Associate Professor, Department of Pharmacology, FH Medical College and Hospital, Agra, Uttar Pradesh, India.
3 Assistant Professor, Department of Pharmacology, FH Medical College and Hospital, Agra, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shivangna Singh, Associate Professor, Department of Pharmacology, FH Medical College and Hospital, Near Etmadpur, Railway Over Bridge, NH-2, Agra-283201, Uttar Pradesh, India.
E-mail: vivu25gtm@gmail.com
Introduction
Alopecia Areata (AA) is a hair disorder characterised by non-scarring, patchy loss of hair from scalp and other parts of the body. For the treatment of AA, topical steroid is one of the first line therapeutic options. Topical vitamin D analogue Calcipotriol has immunomodulatory action. Vitamin D Receptors (VDR) are present in the hair follicles, therefore for treatment of AA topical vitamin D analogue Calcipotriol can be considered.
Aim
To comparatively evaluate the role in terms of efficacy of topical vitamin D analogue Calcipotriol when used along with topical Clobetasol in comparison to topical Clobetasol used alone for AA treatment.
Materials and Methods
In this randomised, open label, clinical study, sixty patients (age 20-32 years) diagnosed with AA were randomly assigned into two groups, thirty patients in each from September 2019 to February 2020. Topical Clobetasol (0.05%) was applied on the affected area twice a day for 24 weeks by Group A patients. While both topical Clobetasol (0.05%) and topical Calcipotriol (0.005%) was applied on the affected area twice daily for 24 weeks by Group B patients. Parametery like Age, Serum Hydroxy Vitamin D (25(OH)D) and Severity of Alopecia Tool (SALT) Score were mesured at baseline. At regular intervals of time (i.e baseline, 6,12,24 weeks), SALT score was evaluated. Mean values of the data were evaluated using student’s t-test and chi-square test based on whether the data was quantitative or qualitative in nature respectively. A p<0.05 was considered statistically significant.
Results
With respect to age and gender distribution both the groups were comparable (p>0.05). For patients of group A and group B the mean values of SALT score at baseline were 10.45±5.25 and 9.85±4.95, respectively (p=0.65). In patients of Group A and Group B towards the end of 24 weeks the mean values of SALT score decreased to 5.98±4.32 (p=0.0007) and 3.66±3.53 (p=0.0001), with a greater decrease in SALT score seen in Group B (p=0.05) i.e., the group in which patients were treated with topical calcipotriol 0.005% along with topical Clobetasol 0.05%.
Conclusion
Topical calcipotriol 0.005% lotion used along with topical Clobetasol 0.05% lotion had higher efficacy than topical Clobetasol 0.05% lotion used alone, in the treatment of AA.
Novel treatment, Severity alopecia tool, Topical steroid, Topical vitamin D analogue
Introduction
The Alopecia Areata (AA) is a common nonscarring, auto-immune, inflammatory disease characterised by patchy loss of hair involving the scalp and other parts of the body [1]. Aetiology behind AA is not clearly known but immune system hypothesis is universally accepted, which states that T-cell induced immune response affects those individulas who are genetically prone [1]. The incidence rate and lifetime risk of AA is 0.1-0.2% and 1.7% respectively [2]. The commencement of this disease can occur in any age group but individuals between 20 to 40 years of age are most commonly affected as majority of patients have first episode of AA in the same age group [2,3]. Although few studies indicated male predominance but both the genders are affected equally [3,4]. Most of the time AA affects scalp (90%) but other parts of the body can also be involved [5].
The treatment of AA depends upon age group of the patient and disease severity. Various drugs like topical and systemic steroids, topical formulations of minoxidil, anthralin and immunosuppressant drugs are used in treatment of AA [6]. The drug of choice for treatment of AA is Corticosteroids because of their anti- inflammatory property. They can be used in both topical and systemic formulations [7].
Intra-lesional injection of corticosteroids like Triamcinolone acetonide is the drug of choice for treatment of adult AA patients with restricted involvement. Most commonly used drug for treatment of patchy AA are topical corticosteroids [6]. Topical preparations in form of cream, lotion, ointment, foam etc of drugs like Clobetasol (0.05%), Betamethasone (0.05%), Fluocinolone (0.2%), Halcinonide (0.1%) etc are widely used in treatment of AA with a sucess rate of 28.5%-61% [7].
Side-effects of topical steroids include folliculitis, skin atrophy, telangiectasia, dermatitis, eczema etc are the common side effects of topical steroids [6]. Topical corticosteroids have a high relapse rate ranging from 37% to 63% after completion as well as during course of treatment [6]. For treatment of AA patients with extensive involvement, systemic corticosteroids are widely used drugs but they lead to wide range of adverse effects like hyperglycemia, acne, weight gain, osteoporosis, cataract, hypertension, Cushing syndrome, immunosuppression etc [6]. Use of systemic steroids has a risk of side-effects and high relapse rate [6].
Vitamin D analog is a novel option for the treatment of AA [8]. Hair follicles have vitamin D receptors (VDRs) which are considered important for normal functioning of hair cycle [9]. Deficiency of these receptors lead to impaired growth and decreased epidermal differentiation of hair follicles [9].
Few studies have demonstrated decreased expression of VDR in hair follicles of area involved [10,11]. Topical application of the vitamin D analogue calcipotriol has also proven to be effective in scalp AA [12,13]. Treatment with topical vitamin D analogue reduced the severity of alopecia and increased hair regrowth rate [12]. Topical vitamin D analogue show better response in patients with vitamin D deficiency, therefore for treatment of vitamin D deficient patients of AA topical Calcipotriol can be used as an alternative [13].
However, there is still paucity of research work especially in Indian population to substantiate the implication of topical vitamin D analogue in treatment of AA. Therefore, this clinical study was conducted. The aim of the study were to evaluate the changes in SALT score at regular intervals of time and to observe any side-effects following the treatment in patients.
Materials and Methods
A randomised control trail was conducted in the Department of Dermatology, Venereology & Leprosy and in Department of Pharmacology of FH Medical College and Hospital, Agra, Uttar Pradesh from September 2019 to February 2020. Study approval was obtained from the Institutional Ethical Committee (IEC/IRB NO. 09/19). Written informed consent was obtained from each participant before starting the treatment after explaining to them about the details related to the drugs to be used in the study.
Inclusion criteria: All clinically diagnosed patients between age group 18 to 65 years, of both the sexes affected with mild to moderate (i.e., less than 50% involvement of entire scalp [14]) patchy AA visiting the Department of Dermatology, Venereology and Leprosy OPD in the time period of the study were made a part of the study according to inclusion criteria.
Exclusion criteria: Patients of age group less than 18 years and more than 65 years, severe AA (i.e., involvement of >50% of entire scalp, alopecia totalis, alopecia universalis [14]), involvement of sites apart from scalp, patients treated with any topical vitamin D analog, patients who were treated with any topical or systemic steroids, immunosuppressive drugs or any other treatment options for AA in the past and nursing mothers and pregnant women were excluded from the study.
Study comprised of a total of sixty patients who were eligible to participate in the study and subsequently separated into two groups by simple randomisation (Group A=30 and Group B=30) [Table/Fig-1]. Group A patients were treated with topical Clobetasol 0.05% lotion alone applied twice daily on the affected area for entire duration of 24 weeks. While patients belonging to Group B applied 0.005% topical calcipotriol lotion along with 0.05% topical clobetasol lotion twice daily on the area affected for 24 weeks. It was an open label study.
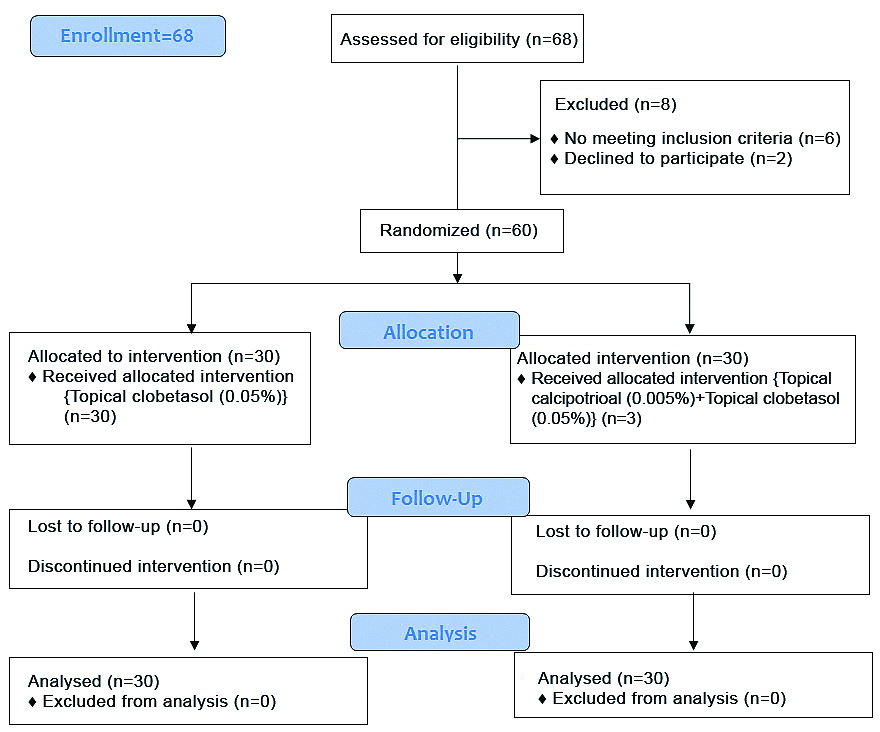
At baseline serum vitamin D levels of the patients included in the study were measured. Quantitative estimation of serum vitamin D levels was done using Enzyme Linked Immunosorbent Assay (ELISA) testing kits was used for quantitative detection of serum 25(OH)D levels [15] during laboratory investigation. SALT score [16] was calculated at baseline and at 6, 12, 24 weeks and patients were also questioned about any side-effect observed following the treatment during each follow-up visit. Patients along with their relatives/guardians accompanying them were questioned and were instructed to bring empty/used and in-use lotion bottles during each follow-up visit for evaluation of treatment compliance.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) version 21.0, IBM, USA was employed for statistical analysis of the data obtained in the study. Student’s t-test was utilized to compare mean values of quantitative data, while Chi-square test was applied to analyse qualitative data. A p-value <0.05 was considered statistically significant.
Results
There were 60 patients in total, with 30 patients in each group. All the patients received the allocated intervention. No patient was lost in follow-up in both the groups.
Gender distribution is displayed in the [Table/Fig-2]. Out of 60 patients enrolled in the study 38 are males and 22 females, male: female ratio is 1.73:1. There was male predominance in each group. Statistically significant difference was not observed within the two groups (p>0.05) [Table/Fig-2].
Gender distribution in both the groups (N=60).
| Parameters | Group A (N=30) | Group B (N=30) | χ2 value | p-value |
|---|
| Male | 20 | 18 | 0.2871 | 0.59 |
| Female | 10 | 12 |
Chi-square test used
In [Table/Fig-3], no significant difference was noted between these two groups with regard to age, serum 25(OH)D levels and SALT score (p>0.05). Patients belonged to age group of 20-32 years of age. All the patients in both the groups had low vitamin D levels (<30 ng/mL).
Baseline parameters of both the groups (N=60).
| Parameters | Group A (N=30) | Group B (N=30) | t-value | p-value |
|---|
| Age (years) | 26.45±4.72 | 27.35±3.56 | 0.8338 | 0.40 |
| Serum25 (OH)D(ng/mL) | 19.89±3.96 | 20.67±4.67 | 0.6977 | 0.48 |
| SALT score | 10.45±5.25 | 9.85±4.95 | 0.4554 | 0.65 |
SALT: Severity of alopecia tool; 25(OH)D: 25 Hydroxy vitamin D; Student t-test used
In [Table/Fig-4], significant decrease (p<0.05) in mean SALT score was observed at 12 weeks and 24 weeks among patients belonging to each group, when compared with baseline value of the same group.
Intra group comparison of SALT score values.
| SALT score (Mean±SD) | Group A (N=30) | Group B (N=30) |
|---|
| Score baseline | 10.45±5.25 | 9.85±4.95 |
| 6 weeks | 8.68±5.04 | 7.57±4.34 |
| (t-value=1.33) | (t-value=1.89) |
| (p-value=0.18) | (p-value=0.06) |
| 12 weeks | 7.40±4.53 | 5.32±3.22 |
| (t-value=2.40) | (t-value=4.20) |
| (p-value=0.01) | (p-value=0.0001) |
| 24 weeks | 5.98±4.32 | 3.66±3.53 |
| (t-value=3.60) | (t-value=5.57) |
| (p-value=0.0007) | (p-value=0.0001) |
Student t-test used; SALT: Severity of alopecia tool
In [Table/Fig-5], At 12 weeks and 24 weeks significant decrease (p<0.05) in SALT score was observed with a higher decrement in group B patients.
Comparison of SALT score between the two groups.
| SALT score (Mean±SD) | Group A (N=30) | Group B (N=30) | t-value | p-value |
|---|
| Baseline | 10.45±5.25 | 9.85±4.95 | 0.4554 | 0.65 |
| 6 weeks | 8.68±5.04 | 7.57±4.34 | 0.9141 | 0.36 |
| 12 weeks | 7.40±4.53 | 5.32±3.22 | 2.0498 | 0.04 |
| 24 weeks | 5.98±4.32 | 3.66±3.53 | 2.2777 | 0.02 |
SALT: Severity of alopecia tool; Student t-test used; bold values are significant
Discussion
A completely novel therapeutic option with the utilisation of topical vitamin D analog calcipotriol in the treatment of AA is emerging [8]. The efficacy of topical analogues of vitamin D in the treatment of AA has been described only in few studies [12,13,16,17]. As there is lack of research to gauge efficacy of topical calcipotriol (0.005%) in the treatment of AA, hence this study was conducted.
Alam M et al, conducted a study to evaluate role of topical Mometasone (0.1%) when used along with topical calcipotriol 0.005% in comparison to use of topical mometasone monotherapy in treatment of AA [17]. SALT score was evaluated during each follow-up visit at 6,12 and 24 weeks [17]. Statistically significant decrease (p-value <0.001) was observed at 24 weeks in mean SALT score in both the groups when compared with the mean baseline score within the same group [17]. It was noteworthy that when difference in the mean SALT score of these two groups was compared together, a statistically significant decrease (p-value <0.001) was found in the group treated with topical 0.1% Mometasone along with Calcipotriol 0.005%.
Similar results were seen in this study where greater decrease in SALT score was seen in the group B. There were no side-effects observed in both the groups, whereas Alam M et al., in their study reported minor side effects in eight patients (four patients in each group) like erythema, dermatitis, folliculitis and atrophy [17].
El-Ghareeb MI., delineated the safety and efficacy of topical preperation of calcipotriol 0.005% mixed with topically applied steroid (betamethasone valerate 0.1%) against topical steroid (betamethasone valerate 0.1%) used alone in treatment of AA [18]. At the end of the treatment duration of three months, highly significant statistical decrease was observed in SALT score values of both the groups. While comparing SALT score changes between the two groups, no statistically significant difference (p=0.88) was observed as both the therapies were equally efficacious. Whereas it was noted in our study that when SALT score between the two groups were compared, a statistically significant difference (p<0.05) was exhibited at 12 weeks and 24 weeks.
The efficacy and safety of topical calcipotriol was evaluated by Cerman AA et al., where it was administered for 12 weeks duration for the treatment of AA [12]. The mean SALT score at week 12 was significantly lower (p-value=0.001). While the present study demonstrated a decrease (p-value=0.0001) in mean SALT score at 12 weeks and 24 weeks duration in the patients who were treated with combination therapy of both topical Calcipotriol and topical Clobetasol and this decrease was statistically significant when compared to baseline SALT score.
The efficacy and safety of treatment with Calcipotriol versus topical Clobetasol 0.05% was studied in a series of thirty five patients with AA affecting the scalp region by Molinelli E et al., [19]. Treatment with calcipotriol ointment showed better, but statistically insignificant, response rates than those treated with topical Clobetasol. As the additional use of topical calcipotriol along with topical steroid provided benefit to the patients in the index study, therefore use of topical vitamin D analogue in treatment of AA shows promise.
Limitation(s)
Limited sample size and short study duration are the limitations.
Conclusion(s)
Topical vitamin D analogue calcipotriol when used along with topical steroid clobetasol as a combination therapy it provides additional benefit in the treatment of AA, therefore it can be used as add on therapy along with topical steroids.
Chi-square test used
SALT: Severity of alopecia tool; 25(OH)D: 25 Hydroxy vitamin D; Student t-test used
Student t-test used; SALT: Severity of alopecia tool
SALT: Severity of alopecia tool; Student t-test used; bold values are significant
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Feb 16, 2021
Manual Googling: Feb 27, 2021
iThenticate Software: Mar 16, 2021 (10%)
[1]. Wadhwa SL, Khopkar U, Nischal KC, Hair and scalp disorders. In: IADVL Textbook of Dermatology 2015 3rd edNew Delhi (India)Bhalani Publishers:903 [Google Scholar]
[2]. Tan E, Tay YK, Goh CL, Chin Giam Y, The pattern of Alopecia Areata in Singapore- A study of 219 AsiansInt J Dermatol 2002 41(11):748-53.10.1046/j.1365-4362.2002.01357.x12452996 [Google Scholar] [CrossRef] [PubMed]
[3]. Sharma VK, Dawn G, Kumar B, Profile of AlopeciaAreata inNorthern IndiaInt J Dermatol 1996 35(1):22-27.10.1111/j.1365-4362.1996.tb01610.x8838924 [Google Scholar] [CrossRef] [PubMed]
[4]. Wasserman D, Guzman-Sanchez DA, Scott K, McMichael A, AlopeciaAreataInt J Dermatol 2007 46(2):121-31.10.1111/j.1365-4632.2007.03193.x17269961 [Google Scholar] [CrossRef] [PubMed]
[5]. Madani S, Shapiro J, Alopecia Areata updateJ Am Acad Dermatol 2000 42(4):549-66.10.1067/mjd.2000.10390910727299 [Google Scholar] [CrossRef] [PubMed]
[6]. Shapiro J, Current treatment of AlopeciaAreataJ Investig Dermatol Symp Proc 2013 16(1):S42-44.10.1038/jidsymp.2013.1424326551 [Google Scholar] [CrossRef] [PubMed]
[7]. Tosti A, Piraccini BM, Pazzaglia M, Vincenzi C, Clobetasol 0.05% underocclusion in the treatment of Alopecia totalis/universalisJ Am Acad Dermatol 2003 49(1):96-98.10.1067/mjd.2003.42312833016 [Google Scholar] [CrossRef] [PubMed]
[8]. Nancy AL, Yehuda S, Prediction and prevention of autoimmune skin disordersArch Dermatol Res 2009 301:57-64.10.1007/s00403-008-0889-318815800 [Google Scholar] [CrossRef] [PubMed]
[9]. Bikle DD, Vitamin D metabolism and function in the skinMol Cell Endocrinol 2011 347(1-2):80-89.10.1016/j.mce.2011.05.01721664236 [Google Scholar] [CrossRef] [PubMed]
[10]. Daroach M, Narang T, Saikia UN, Sachdeva N, Sendhil Kumaran M, Int J Dermatol 2018 57(2):217-22.10.1111/ijd.1385129243839 [Google Scholar] [CrossRef] [PubMed]
[11]. Fawzi MM, Mahmoud SB, Ahmed SF, Shaker OG, Assessment of vitamin D receptors in alopecia areata and androgenetic alopeciaJ Cosmet Dermatol 2016 15(4):318-23.10.1111/jocd.1222427151518 [Google Scholar] [CrossRef] [PubMed]
[12]. Cerman AA, Solak SS, Altunay I, Kucukunal NA, Topical calcipotriol therapy for mild-to-moderate Alopecia Areata: A retrospective studyJ Drugs Dermatol 2015 14(6):616-20. [Google Scholar]
[13]. Narang T, Daroach M, Kumaran MS, Efficacy and safety of topical calcipotriol management of Alopecia Areata: A pilot studyDermatol Ther 2017 30(3):e1246410.1111/dth.1246428133875 [Google Scholar] [CrossRef] [PubMed]
[14]. You HR, Kim SJ, Factors associated with severity of alopecia areataAnnals of Dermatology 2017 29(5):56510.5021/ad.2017.29.5.56528966512 [Google Scholar] [CrossRef] [PubMed]
[15]. Zerwekh JE, The measurement of vitamin D: Analytical aspectsAnnals of Clinical Biochemistry 2004 41(4):272-81.10.1258/000456304120146415298739 [Google Scholar] [CrossRef] [PubMed]
[16]. Bhor U, Pande S, Scoring Systems In DermatologyIndian J Dermatol Venereol Leprol 2006 72:315-21.10.4103/0378-6323.2672216880586 [Google Scholar] [CrossRef] [PubMed]
[17]. Alam M, Amin SS, Adil M, Arif T, Zahra FT, Varshney I, Comparative study of efficacy of topical mometasone with calcipotriol versus mometasone alone in the treatment of Alopecia AreataInt J Trichology 2019 11(3):123-27.10.4103/ijt.ijt_18_1931360041 [Google Scholar] [CrossRef] [PubMed]
[18]. El-Ghareeb MI, Topical calcipotriol mixed with topical steroid versus topical steroid alone in treatment of alopecia areataEgypt J Dermatol Venerol 2019 39(2):78-82.10.4103/ejdv.ejdv_24_18 [Google Scholar] [CrossRef]
[19]. Molinelli E, Campanati A, Brisigotti V, Sapigni C, Paolinelli M, Offidani A, Efficacy and safety of topical calcipotriol 0.005% versus topical clobetasol 0.05% in the management of Alopecia Areata: An intra subject pilot studyDermatol Ther 2020 10(3):515-21.10.1007/s13555-020-00379-732342443 [Google Scholar] [CrossRef] [PubMed]