A 69-year-old female patient with hypertension and diabetes mellitus, was brought to Emergency Department in unresponsive state. The initial evaluation revealed that the patient had wide complex junctional bradycardia owing to hyperkalemia; hypoglycaemia, metabolic acidosis. Treatment was started in an attempt to establish definitive airway; correct glucose levels, cardiac membrane stabilisation. Interim, patient had a cardiac arrest and the Return of Spontaneous Circulation (ROSC) was achieved and then stabilised. Later, she was found to have viral hepatitis complicated with ischaemic hepatitis due to urosepsis. Managing a coding patient with severe metabolic acidosis in a resource limited setting is always challenging. Numerous paradoxes including usage of alkali therapy, choice of inotropes, achievement of haemodynamic neutral intubation is extensively studied yet debated. The present case encompasses the difficulties and possible solutions in managing such patient with refractory acidosis, Bradycardia, Renal failure, Atrioventricular (AV) nodal blockers, Shock, Hyperkalemia syndrome (BRASH).
AV nodal blockers, Bradycardia, Cardiac arrest, Hyperkalemia syndrome, Hyperkalemia, Metabolic acidosis, Renal failure, Shock
Case Report
A 69-year-old female patient was brought into the Emergency in an unresponsive state at around 10:47 am. She was a known hypertensive and diabetic. As per the patient’s son, she was unresponsive for the past one hour. On arrival, she was febrile with temperature of 101°F, her pulse rate was 42 beats per minute (bpm), blood pressure was 104/60 mmHg, saturating at 100% with 4 litres of oxygen through face mask and was hyperventilating with the rate of around 26/min. Capillary blood glucose was 28 mg/dL. Simultaneous evaluation of the clinical history, from her family revealed that, she was on medication which includes amlodipine 5 mg once a day, metoprolol 25 mg once a day and glimiperide 4 mg once a day. She had fever for past 2-3 days with malaise, mild dysuria and had skipped her breakfast on the day.
On examination, she was pale, mildly icteric. Her Glasgow Coma Scale (GCS) was E1V2M3 with a sluggish response of bilateral pupils. Crackles were found at the bases of bilateral chest fields with diffusely reduced breath sounds. Mild hepatosplenomegaly was evident on abdominal examination. There was jugular venous distension noted during examination.
During initial assessment, simultaneous attempts were made to establish intravenous access and 100 mL of 50% dextrose was started. Cardiac monitor revealed junctional bradycardia with the rate varying between 40 to 46 bpm. QRS complexes were wide with duration of 130 milliseconds. Arterial blood was sent for blood gas analysis [Table/Fig-1] and it revealed severe hyperkalemia (6.2 millimols/litre) and high anion gap metabolic acidosis owing to significant lactic acidosis and severe anaemia. A 10% solution of 1 g calcium gluconate was used in an attempt to correct hyperkalaemic changes. Simultaneous reassessment of neurological status was done but there was no improvement in GCS even after dextrose infusion. Decision on intubating the patient was considered. Rapid sequence intubation was done using 40 mg of intravenous atracurium and 3 mg of intravenous midazolam. Potassium reducing measures including 10 mg salbutamol nebulisation and 10 units of intravenous regular insulin- 100 mL of 25% dextrose were given. Calcium gluconate 1 gram intravenously was repeated every 3-5 minutes to reverse the electrocardiographic changes.
Sequential arterial blood gas analysis.
| Timeline | 10:47 On arrival to emergency room | 11:50 63 minutes after arrival | 13:04 137 minutes after arrival | 15:00 253 minutes after arrival | 48 hrs+ |
|---|
| ph of blood | 7.019 | 6.868 | 6.949 | 7.061 | 7.331 |
| pCO2 (mmHg) | 17 | 16.2 | 26.2 | 21.8 | 46.6 |
| pO2 (mmHg) | 106 | 240 | 131 | 119 | 84.2 |
| HCO3 (mEq/L) | 6 | 4.2 | 6.6 | 7.7 | 23.1 |
| Lac (GPL) | 15 | 11 | 14.1 | 12.9 | 1.6 |
| Base excess | -24.8 | -27.7 | -24 | -22.4 | -1.2 |
| Sodium (mEq/L) | 114 | 119 | 115 | 114 | 142 |
| Potassium (mmol/L) | 6.2 | 4.4 | 5.1 | 4.7 | 3.2 |
| Chloride (%) | 92 | 97 | 90 | 91 | 105 |
| Hb (gm/dL) | 6.7 | 6.7 | 6.8 | 6.8 | 10 |
ph: Potential of hydrogen; pCO2: Partial pressure of carbon dioxide; pO2: Partial pressure of oxygen; HCO3: Bicarbonate; Lac: Lupus anti-coagulant; Hb: Haemoglobin
Meanwhile, the patient had a sudden cardiac arrest. Pulse rate was not palpable and cardiac monitor showed asystole. She was revived with a single cycle of cardiopulmonary resuscitation. A single dose of adrenaline 1 mg (1:10000), calcium gluconate 1 gram was used during resuscitation. A total of 5 grams of 10% calcium gluconate was used to reverse the broad complex junctional bradycardia. Arterial line was established for further monitoring of blood pressure and norepinephrine was initiated due to low blood pressure (70/32 mmHg). ROSC care started as per protocol. Ventilatory strategy was selected in a way to have high minute ventilation and low pCO2. Point of Care Ultrasonography (POCUS) revealed dilated inferior vena caval diameter (2.6 cm) with 20% collapsibility, global hypokinesia, B profile as per Bedside Lung Ultrasonography Exam (BLUE) protocol, hepatosplenomegaly [1].
Blood pressure was not maintained with the high support of norepinephrine which was being delivered at 60 mcg/minute. Systolic blood pressure was 80 mmHg but the diastolic blood pressure varied between 10-12 mmHg. Soon, epinephrine infusion was added at 4 mcg/minute. Thereafter, mean arterial pressure was maintained at 65 mmHg with significant increase in mean arterial pressure. Urine output was 10-15 mL/hour in the first two hours.
Haemodialysis line was established under ultrasound guidance through right internal jugular vein. Arterial Blood gas analysis was repeated [Table/Fig-1]. Considering the risk-benefit ratio, 100 mEq of sodium bicarbonate was given over next hour, after alerting the dialysis team to initiate haemodialysis. The patient’s condition deteriorated clinically, requiring increased support of norepinephrine up to 90 mcg/min, epinephrine up to 8 mcg/min [Table/Fig-2]. Repeat Arterial Blood Gas (ABG) analysis after completion of sodium bicarbonate infusion were performed [Table/Fig-1]. Then, haemodialysis was initiated to correct metabolic acidosis. The Electrocardiogram (ECG), blood investigations, ultrasound abdomen, echocardiography, chest radiograph were performed [Table/Fig-3,4 and 5]. Within 4 hours of haemodialysis, her inotrope requirements reduced radically and were weaned off within subsequent 24 hours. Four units of fresh frozen plasma was given during dialysis to correct coagulopathy. Supportive care was offered for the next two days.
Description of events of the patient.
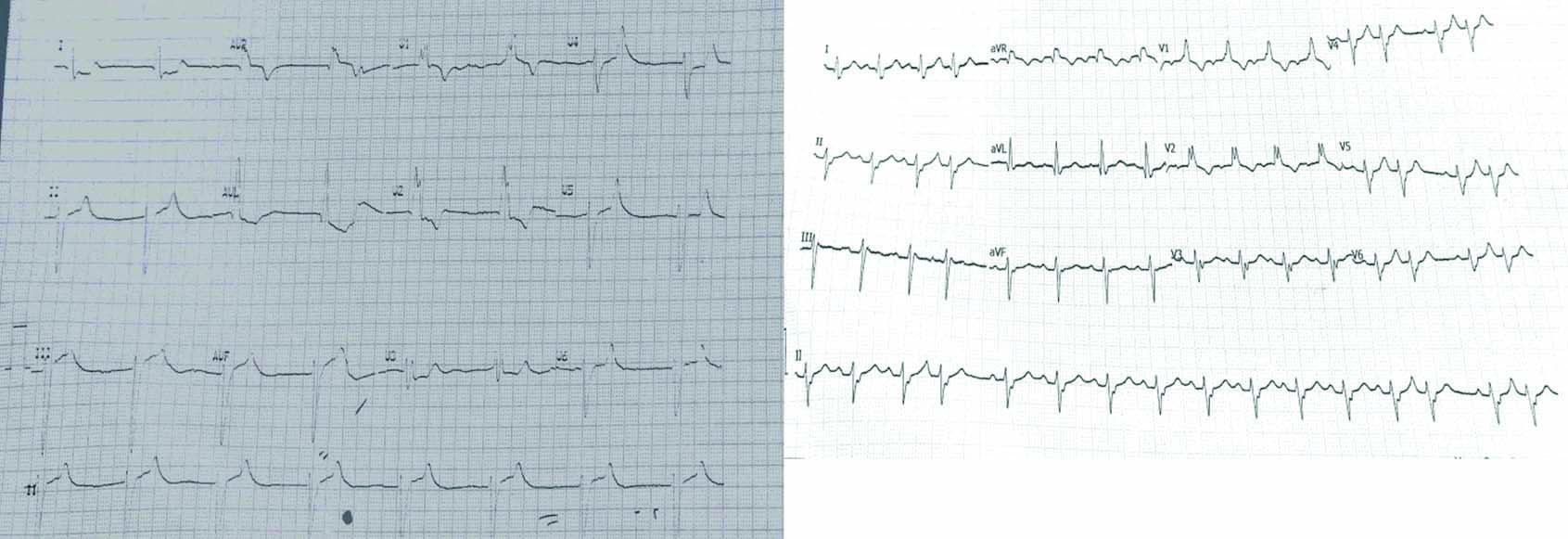
ECG before complete correction of potassium related effects-left side, ECG at 13:00, after correction of potassium related effects-right side.
Chest radiograph, Day 1, Day 2.
| Variables | Values |
|---|
| Haemoglobin, Haematocrit | 6.9 g/dL, 21.6 |
| WBC count | 2800 per microliter |
| Differential count | PMN:78, L:17, E:2, M:3 |
| Peripheral smear | Anisocytosis, Normocytic, Normochromic Anaemia with Leukopenia |
| Platelet count | 180000 per microliter |
| Serum bilirubin | 5.42 mg/dL (Direct: 4,54) |
| SGOT | 6617.8 u/L (N: 10-40) |
| SGPT | 5468.5 u/L (N: 7-50) |
| ALP | 165 IU/L (N: 44-147) |
| Albumin, globulin | 2.8 mg/dL, 3.6 mg/dL |
| INR | 3.95 |
| NT Pro BNP | 3800 pg/mL |
| Dengue NS1, IgM (ELISA) | Negative |
| Malaria dual antigen | Negative |
| Hepatitis B, C, E., HIV | Non-reactive |
| Hepatitis A IgM | Positive |
| Procalcitonin | 1.49 ng/mL (Positive) |
| Ultrasonography | Hepatosplenomegaly, fatty liver |
| Echocardiography (8 hours postcardiac arrest) | No regional wall motion abnormality, good systolic function with 60% ejection fraction, grade 1 left ventricular diastolic dysfunction |
| Blood urea nitrogen | 32 mg/dL |
| Creatinine | 1.8 mg/dL |
| Urine examination | 0-2 pus cells/hpf |
| 2-4 red cells/hpf |
| Albumin: traces |
| Sugar: Nil |
SGPT: Serum glutamic-pyruvic transaminase; SGOT: Serum glutamic-oxaloacetic transaminase; ALP: Alkaline phosphatase; INR: International normalised ratio; NT Pro BNP: N-terminal pro b-type natriuretic peptide; WBC: White blood cell; IgM: Immunoglobulin M
On day 7, she was discharged in stable condition without neurological deficit. Fulminant hepatitis due to Hepatitis A, urosepsis, BRASH syndrome were the diagnoses of the patient that caused refractory acidosis and cardiac arrest on arrival.
Discussion
Bradycardia, renal failure, AV nodal blockers, shock, hyperkalemia: In this case, the electrocardiographic changes [Table/Fig-2] pertaining to hyperkalemia was resistant to multiple dosing of calcium gluconate. Hyperkalemia worsened due to acidosis, concomitant usage of beta blocker in the setting of acute kidney injury can cause the decreased clearance of them. This will have the synergistic effect causing refractory bradycardia [2,3]. Continuous dosing of calcium gluconate can augment the existent vasodilation worsening the overall scenario. On the other hand, risk of extravasation of calcium chloride if given in smaller veins is the limitation for calcium chloride usage.
Ventilating a patient with severe metabolic acidosis: Even a subtle amount of apneic period is intolerable for a patient with severe metabolic acidosis. The usual protocol of stopping bag ventilation after inducing, paralysing in rapid sequence intubation may be flawed in this setting. Bagging has to be continued at the risk of mild aspiration. The intracellular acidosis in this setting, must have had significantly compromised inotropy causing the cardiac arrest for our patient [4]. Realising the scenario, the aggressive ventilation strategy was adapted targeting high minute ventilation to maintain the near similar pCO2 levels as before.
Choice of epinephrine as a pressor in the setting of hyperlactatemia: Epinephrine acts on G protein coupled receptors to stimulate sodium-potassium ATPase leading to further breakdown of Adenosine tri-phosphate (ATP) and generation of Adenosine di-phosphate (ADP) which will re-stimulate the glycolysis. This mismatch indirectly augments lactate production [4,5]. The key to this equation is that the lactate production from pyruvate consumes protons which ultimately causes alkalosis [6,7]. So, the clinicians cannot afford to miss a potential drug which has inotropic, vasoconstrictor property which is the need of the hour.
Sodium bicarbonate infusion in cardiac arrest scenario: Multiple studies have reiterated the potential deleterious effects of sodium bicarbonate post-ROSC. In this case, the acidosis seen post-ROSC was evident even before cardiac arrest. Cardiac arrest contributed to the worsening of already acidotic status of the patient. So, sodium bicarbonate was given considering the risks involved. Within half an hour after starting sodium bicarbonate, the inotrope requirement of the patient gradually increased. Arterial Blood Gas (ABG) analysis done after an hour showed improvement in pH but the clinical status deteriorated.
The buffering effect of bicarbonate can only be expected if the pH is in between 5.1 to 7.1 as the pKa of bicarbonate is 6.1 [8]. Nevertheless, subsequent release of carbon dioxide intracellularly causes paradoxical acidaemia, worsening the clinical status [9,10]. Sodium bicarbonate infusion might cause temporary increase in pH extracellularly but decreases the intracellular pH [11,12]. Sodium bicarbonate infusion causes the left shift of oxygen dissociation curve causing difficulty in unloading oxygen to the tissue, further complicating the scenario. Rapid infusion of hyperosmolar solutions like hypertonic saline, bicarbonate can cause solvent drag phenomenon including potassium imbalance, worsening the existent hyperkalemia, which is usually expected in the setting of acidosis [13]. So, in this case, despite better pH in subsequent blood gas analysis after bicarbonate infusion, the inotrope requirement was not reduced.
Probable disease course in the present case: Hypoglycaemia initially masked the seriousness of the condition. Hepatitis A and subsequent fulminant hepatitis complicated the patient with urosepsis. Increased lactates produced in the setting of sepsis were not cleared due to liver failure, causing significant hyperlactatemia. Fulminant hepatitis indeed causes changes in the systemic vascular resistance and arteriovenous shunting augmenting already developed lactic acidosis [14]. Early initiation of haemodialysis may not be a possibility in all the settings in developing countries. Utilising the golden hour with medical management is of obvious need.
Conclusion(s)
Managing a patient with severe metabolic acidosis, hyperkalemia in a resource limited setting is complicated. Correction strategies including sodium bicarbonate is highly debated. This case report tried to dissect the obvious limitations involved in the treatment and presented possible solutions.
ph: Potential of hydrogen; pCO
2: Partial pressure of carbon dioxide; pO
2: Partial pressure of oxygen; HCO
3: Bicarbonate; Lac: Lupus anti-coagulant; Hb: Haemoglobin
SGPT: Serum glutamic-pyruvic transaminase; SGOT: Serum glutamic-oxaloacetic transaminase; ALP: Alkaline phosphatase; INR: International normalised ratio; NT Pro BNP: N-terminal pro b-type natriuretic peptide; WBC: White blood cell; IgM: Immunoglobulin M
[1]. Daniel A Lichtenstein, Gilbert A Mezière, Relevance of Lung Ultrasound in the Diagnosis of Acute Respiratory Failure: The blue protocolChest 2008 134:117-25.10.1378/chest.07-280018403664 [Google Scholar] [CrossRef] [PubMed]
[2]. Rawal KB, Chhetri DR, Giri A, Girish HN, Luhar MB, Anusha S, Metoprolol-induced hyperkalemia- A case reportIndian J Med Sci10.25259/IJMS_134_2020 [Google Scholar] [CrossRef]
[3]. Hawboldt J, McGrath D, Possible metoprolol-induced hyperkalemiaJ Pharm Pract 2006 19(5):320-25.10.1177/0897190007300728 [Google Scholar] [CrossRef]
[4]. Downing SE, Talner NS, Gardner TH, Cardiovascular responses to metabolic acidosisAm J Physiol-Leg Content 1965 208(2):237-42.10.1152/ajplegacy.1965.208.2.23714259953 [Google Scholar] [CrossRef] [PubMed]
[5]. Belletti A, Nagy A, Sartorelli M, Mucchetti M, Putzu A, Sartini C, Effect of continuous epinephrine infusion on survival in critically ill patients: A meta-analysis of randomized trials*Crit Care Med 2020 48(3):398-405.10.1097/CCM.000000000000412731789701 [Google Scholar] [CrossRef] [PubMed]
[6]. Robergs RA, Invited review: Quantifying proton exchange from chemical reactions- Implications for the biochemistry of metabolic acidosisComp Biochem Physiol A Mol Integr Physiol 2019 235:29-45.10.1016/j.cbpa.2019.04.02431071454 [Google Scholar] [CrossRef] [PubMed]
[7]. Green A, Bishop RE, Ketoacidosis- Where do the protons come from?Trends Biochem Sci 2019 44(6):484-89.10.1016/j.tibs.2019.01.00530744927 [Google Scholar] [CrossRef] [PubMed]
[8]. Sood P, Paul G, Puri S, Interpretation of arterial blood gasIndian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med 2010 14(2):57-64.10.4103/0972-5229.6821520859488 [Google Scholar] [CrossRef] [PubMed]
[9]. Goldsmith DJ, Forni LG, Hilton PJ, Bicarbonate therapy and intracellular acidosisClin Sci Lond Engl 1979 1997 93(6):593-98.10.1042/cs09305939497798 [Google Scholar] [CrossRef] [PubMed]
[10]. Forni LG, Hodgson LE, Selby NM, The Janus faces of bicarbonate therapy in the ICU: Not sure!Intensive Care Med 2020 46(3):522-24.10.1007/s00134-019-05885-731820031 [Google Scholar] [CrossRef] [PubMed]
[11]. Stacpoole PW, Lactic acidosis: The case against bicarbonate therapyAnn Intern Med 1986 105(2):276-79.10.7326/0003-4819-105-2-276 [Google Scholar] [CrossRef]
[12]. Drumheller BC, Sabolick EE, Hemodynamic instability and abnormal vasopressor responsiveness in the setting of severe metabolic acidosis treated with adapted alkalinization and continuous renal replacement therapy in the emergency departmentJ Emerg Med 2020 Oct :S073646792030957410.1016/j.jemermed.2020.09.02233875156 [Google Scholar] [CrossRef] [PubMed]
[13]. Conte G, Dal Canton A, Imperatore P, De Nicola L, Gigliotti G, Pisanti N, Acute increase in plasma osmolality as a cause of hyperkalemia in patients with renal failureKidney Int 1990 38(2):301-07.10.1038/ki.1990.2002402122 [Google Scholar] [CrossRef] [PubMed]
[14]. Bihari D, Gimson AE, Lindridge J, Williams R, Lactic acidosis in fulminant hepatic failure. Some aspects of pathogenesis and prognosisJ Hepatol 1985 1(4):405-16.10.1016/S0168-8278(85)80778-9 [Google Scholar] [CrossRef]