The sphenoid bone is a key bone of the neurocranium. It articulates with several other bones, and it functions as a main pillar for the cranial vault. It possesses three small paired apophyses on its cranial surface: the anterior, middle, and posterior clinoid processes. These bony prominences are known as attachment points of dura-mater extensions [1]. A known variant of the sphenoid bone is the CCF, which is formed by a bony bridge (the caroticoclinoid bar) between the anterior and middle clinoid processes. This foramen gives passage to the internal carotid artery [1,2].
This variation is relevant to surgical access to the cavernous sinus and the paraclinoidal region, especially in cases of tumoural resection or vascular pathologies. Its removal may be necessary during endoscopic, endonasal approaches. Furthermore, fracture of this osseous bar or iatrogenic injuries during anterior or middle clinoidectomies can lead to internal carotid artery rupture, while this bar itself may cause compression of the aforementioned vessel [3-5].
The CCF can be classified into three different types: 1) the complete type, in which there is a full foramen or bony ring between the processes, without any line or suture; 2) the contact type, in which there is a suture or a line between the processes; or 3) the incomplete type, in which there is a bony extension between the structures, but there is no contact [6,7].
Studies have shown significantly different data regarding its prevalence. For instance, a study conducted by Brahmbhatt RJ et al., [8] showed a prevalence of 6%, while a study conducted by Efthymiou E et al., [5] showed a prevalence of 73%, which highlights regional differences [6]. There is a lack of studies regarding its prevalence in South American skulls [9]. Thus, the present study aims to observe the prevalence and size of the CCF in Brazilian dry skulls and discuss its surgical and clinical significance.
Materials and Methods
This was an observational cross sectional study carried out for a period of 10 months during March 2019 to January 2020. The material was obtained from two Anatomy Departments of two Universities situated in the Southeast region of Brazil. Convenience sampling was used in this study. All dry skulls from these departments were assessed with the purpose of verifying the prevalence and size of the CCF. Several skulls were excluded due to damage on the sphenoid bone, and as such, the total sample size was composed of 365 dry skulls.
The presence, side, and size of the CCF were observed and measured with a digital vernier caliper (0.01 mm precision). The CCF was classified into three different types: 1) the complete type, 2) the contact type, 3) the incomplete type according to the literature, as previously described [7].
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism 6 software, the means were analysed by the two-tailed student t-test. The frequency by side and gender differences were analysed by the chi-square test (p-value <0.05 was considered significant for both analyses). Data were presented as mean with (±) standard deviation and/or as percentage.
Results
The CCF was observed in 101 skulls of 365 (a prevalence of 27.6% in the studied sample). Most of the skulls had a bilateral CCF (88 cases of 101 individuals). In the unilateral cases (13 cases), it was observed that 7 were situated on the right side, while the remaining 6 were situated on the left side [Table/Fig-1] (p>0.05). Regarding its classification, out of 190 CCF analysed, 69 were pertaining to the complete type (36.3%), while 120 cases were classified as incomplete (63.1%) and 1 from the contact type (0.6%).
Photograph displaying the caroticoclinoid foramen in the left side and the trajectory of the internal carotid artery (red arrow).
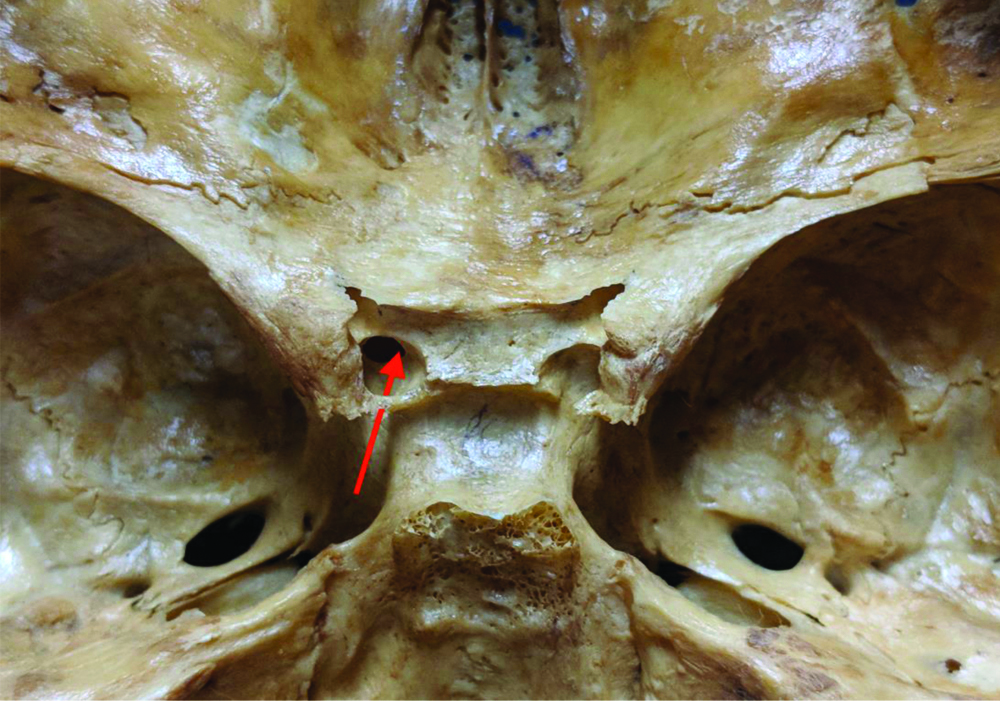
The mean anteroposterior diameter and the TD showed no statistical significance regarding sides (p>0.05). Of 101 skulls with the CCF, 69 had information regarding sex and age. The age ranged from one-month-old to 104-year-old (mean of 37.79±21.85). It was observed that 23 of them were from women (33.3%), while 46 were from men (66.7%). This difference was statistically significant (p<0.05). All these data are summarised in [Table/Fig-2].
Prevalence and size of the Caroticoclinoid Foramen (CCF) in the current study.
| Total (365 skulls) |
|---|
| CCF | 101 (27.6%) | p-value |
| Male | 46 (66.7% of 69) | p<0.05* |
| Female | 23 (33.3% of 69) |
| Size/Side | Right | Left |
| APD (mm) | 4.87±0.69 | 4.86±0.79 |
| TD (mm) | 4.85±0.75 | 4.74±0.73 |
| p-value | p>0.05 |
CCF: caroticoclinoid foramen; APD: Anteroposterior diameter; TD: Transverse diameter; *p-value of <0.05 was considered significant Statistical analysis: GraphPad Prism 6 software, two-tailed student t-test, chi-square test
Discussion
The CCF was thought to be firstly described by Henle in 1885, thus leading to the eponym “carotico clinoid foramen of Henle” [2]. However, recent research showed that this anatomical variant was firstly described by Alexander Monro (primus), in 1726 [10].
To date, there is still mystery regarding its origin, although the most probable theory is that this variant occurs after the ossification of a dural fold between the anterior clinoid process and middle clinoid process [2,6]. An associated theory is that CCF’s formation is related to the embryologic chondrocranium’s developmental anomalies, which is a complex process mediated by several proteins that can slow down or speed up the ossification process [11].
Some authors believed that the formation of the CCF was influenced by age, as it was thought to be more commonly found in individuals over 50-year-old and as such, it was a product of aging itself [12,13]. However, this is not entirely accurate, as the study conducted herein observed the CCF in a one-month and a five-month-old individual. Furthermore, other papers have also shown the presence of the CCF in foetal and infant skulls [3,14,15].
The prevalence of the CCF in the present study was 27.6%, in contrast with another study performed in Brazil, which showed a prevalence of 8.5% in 80 skulls, also from the Southeast region [9]. These results highlight the regional differences within the same country. Another example can be found in the study performed in Turkey by Ozdogmus O et al., [6], which showed a prevalence of 5.5%, while another study showed a prevalence of 35.7%, also from a Turkish sample [13]. An Indian study showed a prevalence of 37.1% out of 223 skulls [16], while another study from India observed a prevalence of 14.5% in 200 dry skulls [17]. In contrast, a study showed a CCF prevalence of 60.1% in 123 skulls from Greece [18], while another observed a prevalence of 74% in 76 Greek dry skulls [5]. This data is presented in [Table/Fig-3] [3,5,6,8,9,13,16-18].
Prevalence of the Caroticoclinoid Foramen (CCF) in several studies.
| Author/Year | Country | Sample size (n) | Prevalence |
|---|
| Ozdogmus O et al., [6] | Turkey | 50 | 5.5% |
| Erturk M et al., [13] | Turkey | 171 | 35.7% |
| Desai S and Sreepadma S [16] | India | 223 | 37.1% |
| Kanjiya D et al., [17] | India | 200 | 14.5% |
| Brahmbhatt R et al., [8] | India | 50 | 6% |
| Jha S et al., [3] | India | 108 | 22.2% |
| Natsis K et al., [18] | Greece | 123 | 60.1 |
| Efthymiou E et al., [5] | Greece | 76 | 74% |
| Freire A et al., [9] | Brazil | 80 | 8.5% |
| Present study | Brazil | 365 | 27.6% |
As such, it can be seen that the prevalence of the CCF is highly variable when different populations or even different regions from the same country are put in perspective. A recent meta-analysis showed that the pooled prevalence of the CCF was 32.6% out of 27 studies (a total of 14.449 skull sides were evaluated), a relatively high prevalence of a clinically significant variation [2].
The present work also corroborates the hypothesis that other parameters such as age, genetic and molecular factors play a significant role in the development of the ligament ossification and the origin of the CCF [5,18].
The study performed by Boyan N et al., observed a bilateral presence of the CCF in 29.4% of their sample [19], while the study conducted by Natsis K et al., showed a bilateral presence of 17.9% [18] and the study by Souza A et al., showed a prevalence of 9.2% [4]. To date, the present study showed the highest bilateral presence of the CCF (88 cases) in a significantly large sample. Several authors also pointed out that the bilateral presence of the CCF was more common than the unilateral presence of this variant [5,13,17,20], while others have stated otherwise [2]. Moreover, in cases where it is unilateral, studies have shown that it has a predilection for the right side, which was observed in the present research, although with no significant difference [9,18].
Authors observed a significant difference in the prevalence between men and women (66.7% vs. 33.3%), which could indicate that it is indeed more common in males. This is in agreement with other studies in the literature [21], although some studies showed otherwise [9]. In opposition, the work performed by Jha S et al., and Ozdogmus O et al., showed no correlation whatsoever between the prevalence of CCF and sex [3,6].
According to the type, the percentage found in this study was higher for the incomplete type (63.1%) in comparison to the complete type (36.3%). Other authors showed that the incomplete type was more common as well [3-5]. This is in conflict with the results observed by Ozdogmus O et al., which showed a prevalence of 27% for the complete type, 18% for the incomplete type and 55% for the ligamentous type, after performing 50 autopsies [6]. The data presented in the present study, was not reliable regarding this matter, as this analysis was performed in dry skulls, as such, the ligamentous type could not be investigated. Authors observed a unilateral CCF of the contact type, which has been signalled in the literature as the rarest type [5] and in accordance to the results published by Jha S et al., (2017) [3].
Regarding the size of the CCF, we observed a mean anteroposterior diameter of 4.87±0.69 and 4.86±0.79 mm for the right and left sides, respectively, Natsis K et al., observed an anteroposterior diameter of 5.41±0.74 and 5.35±1.26 for the right and left sides, higher values than the ones observed herein [18].
The TD observed in the present study was 4.85±0.75 and 4.74±0.73 for the right and left sides, respectively. Efthymiou E et al., showed a TD of 5.40±0.80 and 5.60±0.70 mm [5], Natsis K et al., observed a TD of 5.32±0.54 and 5.56±0.9 mm [18], while Ertürk M et al., study showed a TD of 5.33±0.3 and 5.32±0.73 [13]. In a Brazilian sample, Freire A et al., described a mean of 5.18±4.66 and 5.35±0.71 for the right and left sides, respectively [9]. These values show a significantly larger CCF than the ones observed in the present work, although we highlight the fact that some of the skulls analysed herein were from infants, which could contribute to the smaller size of the CCF in general.
In respect to its clinical significance, the complete CCF can lead to compression of the cavernous sinus and its content, such as the abducens nerve or the internal carotid artery, especially in cases of pneumatisation of the anterior clinoid process [4,18]. Furthermore, it can lead to more difficulty in exposing the paraclinoid region when facing an aneurysm. Some authors noted that tightening or compression of the internal carotid artery could cause headaches as well [22].
It is highly important for the surgeon to identify the CCF preoperatively since the retraction of the cavernous segment of the internal carotid artery could lead to tear or rupture of this vessel and cause significant haemorrhage [21]. In such cases, the surgeon should opt for an intradural approach to better visualise the internal carotid artery. However, recognition of this variation by imaging exams is not entirely accurate, as shown by Skandalakis G et al., as such the surgeon should always be vigilant of the CCF [2].
Limitation(s)
The present study was limited in the sense that it did not approach cadaveric specimens; as such it was not able to observe the ligamentous caroticoclinoid bar.
Conclusion(s)
The CCF can be a significantly common finding based on specific populations, especially when dealing with males, although it may vary even in certain regions of a same country. This study reinstates the CCF high prevalence, indicating that its presence should always be predicted when dealing with surgeries around the paraclinoidal area. Its misidentification is involved with an unfavourable prognosis, since iatrogenic injury of the internal carotid artery can lead to significant haemorrhage. Therefore, preoperative vigilance of such area through imaging exams is highly recommended. Authors believe that further studies dealing with this variant should investigate the formation of this variant during fetal development to better understand its origin.
CCF: caroticoclinoid foramen; APD: Anteroposterior diameter; TD: Transverse diameter; *p-value of <0.05 was considered significant Statistical analysis: GraphPad Prism 6 software, two-tailed student t-test, chi-square test