The term Diabetes mellitus describes a metabolic disorder of multiple aetiologies characterised by chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action or both. The effect of diabetes mellitus includes a long-term damage, dysfunction and failure of various organs [1].
Bromocriptine is a D2-dopamine agonist, that has been recently approved for treatment of type II diabetes [2]. This centrally acting anti-diabetic agent has a novel mechanism of action by decreasing peripheral insulin resistance. Sitagliptin is an orally potent, selective Dipeptidyl Peptidase-4 (DPP-4) inhibitor with excellent oral bioavailability and efficacy in increasing insulin secretion [3].
Sitagliptin, which increases the insulin secretion from the pancreatic beta cells and Bromocriptine, which decreases the peripheral insulin resistance, thus increasing the insulin sensitivity of the receptors, might provide a better plasma glucose control when given together. Even though, both drugs are well studied as monotherapy, the possible synergism has not been established yet. The purpose of this study was to evaluate the synergistic effect of these drugs, if any, in the control of blood sugar in type II diabetes mellitus.
Materials and Methods
This was an experimental animal study conducted in Sri Manakula Vinayagar Medical College and Hospital, Pondicherry, India. Forty-eight adult male albino wistar rats were selected for the experiment. Rats were divided randomly into six groups. Each group had eight rats. This study was conducted for a period of one month (August 2014).
Before starting the experiment, the blood glucose was measured using glucometer strip (Sugar check). The rats were rendered diabetic by a single intraperitoneal injection of 40 mg/kg [4] Streptozotocin (STZ) freshly dissolved in ice-cold 0.1 M citrate buffer (pH 4.5) and injected within 10 minutes after preparation [5].
Three weeks after STZ injection, overnight fasting blood samples were collected from the tail vein of all animals and the baseline serum glucose concentrations, lipid profile (Total cholesterol, High Density Lipoproteins (HDL), Low Density Lipoproteins (LDL), Very Low Density Lipoprotein (VLDL), Liver enzymes Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT)) and Kidney function tests (Blood urea, creatinine) were measured. Only those animals with blood glucose level higher than 200 mg/dL were selected for the study. Rats with blood glucose levels exceeding 400 mg/dL were administered 10 units of insulin subcutaneously. Rats were grouped as follows and given their respective treatment. As the combination’s safety was not assessed in previous studies, three grades of combination therapy doses were administered.
Grouping of animals:
Group I: Vehicle (Distilled water)
Group II: Sitagliptin (100 mg/kg) [6] p.o (per oral)
Group III: Bromocriptine (10 mg/kg) [7] p.o
Group IV: Sitagliptin (75 mg/kg)+Bromocriptine (7.5 mg/kg) p.o
Group V: Sitagliptin (100 mg/kg)+Bromocriptine (10 mg/kg) p.o
Group VI: Sitagliptin (125 mg/kg)+Bromocriptine (12.5 mg/kg) p.o
All the above drugs were given once daily. After 4 weeks of treatment, the parameters shown above were measured again. At the end of the study, the animals were sacrificed with high doses of phenobarbitone. Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines were adhered and Institutional Animal Ethics Committee clearance (SMVMCH/IAEC/DIR/O.NO 10-17/10/12) was obtained before the research.
Statistical Analysis
Statistical analysis was performed using SPSS software version 16.0. Data was analysed by one-way Analysis of Variance (ANOVA) and results were expressed as mean±Standard Deviation (SD). Significance of difference between groups were further analysed with Dunnett’s (2-sided) test for post-hoc comparisons. The p-value of <0.05 was considered statistically significant.
Results
a. Effect on body weight and fasting blood sugar
The vehicle treated animals showed a mean body weight of 154±18.51 g and fasting blood sugar of 347.25±61.71 mg/dL. Oral administration of Sitagliptin (100 mg/kg) and Bromocriptine (10 mg/kg) alone showed a significant (p<0.05) decrease in body weight (137.25±30.69, 125±28.31, respectively). The Sitagliptin and Bromocriptine treated animals also showed a significant decrease in fasting blood sugar levels (p<0.001) [Table/Fig-1].
Effect of Sitagliptin and Bromocriptine (alone and in combination) on fasting blood sugar values of diabetic albino wistar rats.
*p<0.05, **p<0.01, ***p<0.001 as compared with vehicle group; #p<0.05, ##p<0.01, ###p<0.001 as compared with Sitagliptin group; $p<0.05, $$p<0.01, $$$p<0.001 as compared with Bromocriptine group; Comparison was done by one way-ANOVA followed by Post-Hoc Dunnett’s (2-sided) test
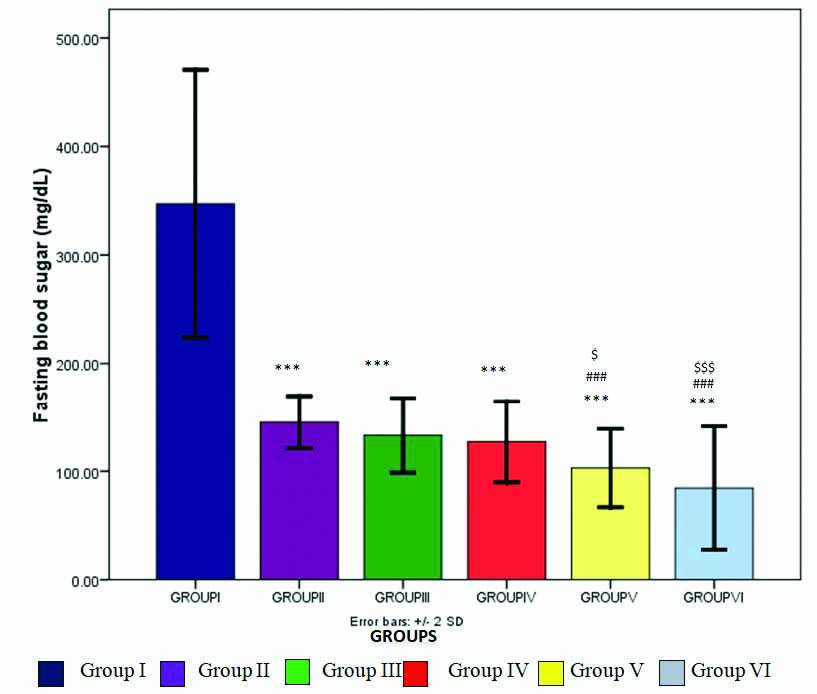
b. Effect on lipid profile
The combination of Sitagliptin and Bromocriptine at different doses presented a favourable effect on lipid profile than the monotherapy and vehicle treated group. The low dose combination (Sitagliptin-75 mg/kg+Bromocriptine-7.5 mg/kg) decreased the total cholesterol (p<0.01), LDL (p<0.05) and VLDL (p<0.01) levels than the vehicle treated animals as depicted in [Table/Fig-2]. The normal dosage combination (Sitagliptin-100 mg/kg+Bromocriptine 10 mg/kg) also showed a similar effect of low dosage combination therapy {Total cholesterol (p<0.01), LDL (p<0.05) and VLDL levels (p<0.001)}. Moreover, the high dose combination (Sitagliptin-125 mg/kg+Bromocriptine 12.5 mg/kg) decreased the total cholesterol (p<0.001), LDL (p<0.001) and VLDL values (p<0.001) which was highly statistically significant. The HDL values remain unchanged in all the combination therapy group animals as compared to the vehicle treated animals.
Effect of Sitagliptin and Bromocriptine (alone and in combination) on fasting lipid profile values of diabetic albino wistar rats.
| Group | Treatment (mg/kg, oral) | Total Cholesterol (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) |
|---|
| Group I | Vehicle | 71.2±10.9 | 27.0±7.6 | 12.9±2.7 | 31.6±9.9 |
| Group II | Sitagliptin (100) | 85.9±13.8 | 50.5±11.7*** | 10±2 | 25.3±6.4 |
| Group III | Bromocriptine (10) | 67.0±19.0## | 39.5±15.7* | 11.4±3.7 | 16.1±6.6*** ## |
| Group IV | Sitagliptin (75)+Bromocriptine (7.5) | 52.8±7.0** ###$ | 24.5±3.3 | 8.2±4.0* | 20.1±7.1** |
| Group V | Sitagliptin (100)+Bromocriptine (10) | 48.6±7.1** ###$ | 26.5±3.7 | 8.37±1.2* | 11.3±4.2*** ### |
| Group VI | Sitagliptin (125)+Bromocriptine (12.5) | 39.1±3.1*** ###$$$ | 23.8±3.9 | 5.75±0.8*** $$ | 9.5±3.3*** ### |
*p<0.05, **p<0.01, ***p<0.001 as compared with vehicle group; #p<0.05, ##p<0.01, ###p<0.001 as compared with Sitagliptin group; $p<0.05, $$p<0.01, $$$p<0.001 as compared with Bromocriptine group; Comparison was done by one way-ANOVA followed by Post-Hoc Dunnett’s (2-sided) test; HDL: High density lipoproteins, LDL: Low density lipoproteins, VLDL: Very low density lipoprotein
c. Effect on renal parameters
In groups treated with Sitagliptin (100 mg/kg) and Bromocriptine (10 mg/kg) monotherapy, there were no significant alteration in blood urea and creatinine levels as compared to the untreated vehicle group. The lower dose combination (Sitagliptin-75 mg/kg+Bromocriptine 7.5 mg/kg) treated animals showed a highly significant decrease in blood urea compared to vehicle, Bromocriptine and Sitagliptin monotherapy treated animals (p<0.001). Combination of Sitagliptin and Bromocriptine (100 mg/kg+10 mg/kg, respectively) also showed a significant (p<0.01) reduction in blood urea levels. And at increased doses (Sitagliptin 125 mg/kg+Bromocriptine 12.5 mg/kg) no significant change was noted in the urea and creatinine levels.
d. Effect on liver parameters
Administration of Sitagliptin (100 mg/kg) and Bromocriptine (10 mg/kg) orally showed a reversal of AST/ALT ratio of 0.9 (AST-217.9±23.4, ALT-237.8±37.9) and 0.7 (AST-150.6±26, ALT-207.5±28.6) respectively as shown in [Table/Fig-3].
Effect of Sitagliptin and Bromocriptine (alone and in combination) on liver parameters of diabetic albino wistar rats.
| Group | Treatment (mg/kg, oral) | AST (IU/L) | ALT (IU/L) |
|---|
| Group I | Vehicle | 264.6±63.7 | 77.7±17.3 |
| Group II | Sitagliptin (100) | 217.9±23.4* | 237.8±37.9*** |
| Group III | Bromocriptine (10) | 150.6±26.0***### | 207.5±28.6*** |
| Group IV | Sitagliptin (75)+Bromocriptine (7.5) | 149.2±27.7***### | 187.8±20.8***## |
| Group V | Sitagliptin (100)+Bromocriptine (10) | 173.5±12.3***### | 201.5±25.5***# |
| Group VI | Sitagliptin (125)+Bromocriptine (12.5) | 173.7±8.9***### | 213±10.7*** |
Values are expressed as Mean±SD for all six groups (n=8 in each group); *p<0.05, **p<0.01, ***p<0.001 as compared with vehicle group; #p<0.05, ##p<0.01, ###p<0.001 as compared with Sitagliptin group; $p<0.05, $$p<0.01, $$$p<0.001 as compared with Bromocriptine group.
Comparison was done by one way-ANOVA followed by Post-Hoc Dunnett’s (2-sided) test; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase
The AST/ALT ratio reversal due to the combination therapy was found to be lesser than the monotherapy. In groups IV, V and VI the change in liver parameters was similar. Low dose combination (Sitagliptin-75 mg/kg+Bromocriptine-7.5 mg/kg) showed a ratio of 0.8 (AST-149.2±27.7 and ALT-187.8±20.8). The normal dose combination (Sitagliptin-100 mg/kg+Bromocriptine-10 mg/kg) produced an AST/ALT ratio of 0.86 (AST-173.5±12.3, ALT-201.5±25.5). With high dose combination therapy (Sitagliptin-125 mg/kg+Bromocriptine-12.5 mg/kg) the ratio was maintained at 0.8 (AST-173.7±8.9 and 213±10.7).
Discussion
This study evaluated the synergistic effect of Sitagliptin and Bromocriptine in STZ induced diabetic albino wistar rats. Adding these drugs together resulted in significant improvements in glycaemic control than vehicle treated, and animals treated with Sitagliptin and Bromocriptine monotherapy.
Fasting blood sugar: The present study suggested that Sitagliptin and Bromocriptine monotherapy significantly decreased the fasting blood sugar compared to vehicle treated group. Reduction in blood sugar levels was like the study conducted by Ferreira L et al., who showed that Sitagliptin monotherapy significantly improved the glycaemic control compared with the vehicle treated animals (p<0.001) [8]. Harish Kumar VS et al., showed a significant decrease in blood sugar (p<0.001) with Bromocriptine monotherapy [9].
Moreover, the combination of Sitagliptin and Bromocriptine also significantly decreased fasting blood sugar compared to vehicle group. This combination (Sitagliptin+Bromocriptine) compared to Sitagliptin and Bromocriptine monotherapy showed a highly significant decrease in fasting blood sugar in a dose dependent manner (p<0.001). Optimal decrease in fasting blood sugar was observed with low and normal doses. However, at high dose combination, most of the rats were found to be hypoglycaemic (n=5/8 animals). This profound decrease could be due to the additive effect of these two drugs given in higher doses, as this hypoglycaemic effect was not seen in low and normal dose combination therapy.
Body weight: The decrease in body weight was observed with Sitagliptin and Bromocriptine combination therapy at three different doses compared to vehicle treated animals (Low dose combination-p<0.05, normal and high dose combination-p<0.01). Sitagliptin, when given alone, was found to decrease the body weight significantly (p<0.05) though Ishii H et al., Mu J et al., Lee YK et al., reported an insignificant decrease in body weight with Sitagliptin [10-12]. Piji H et al., showed that there was no significant change in the body weight with Bromocriptine monotherapy [2]. The results of this study, which goes in conjunction with studies conducted by Shivaprasad C and Kalra S, and Mahajan R represented a significant decrease (p<0.05) in body weight with Bromocriptine monotherapy [13,14]. The decrease in body weight due to Bromocriptine could be due to resetting of circadian dopaminergic and serotonergic activities that regulate the dramatic seasonal alterations in body weight and body compositions of vertebrate classes [15].
Lipid profile: Increase in LDL, VLDL and total cholesterol often results in increased risk of atherosclerosis. It is well known that increased HDL levels have a protective role in CAD. Bromocriptine monotherapy showed a significant decrease in VLDL levels. The HDL levels were observed to rise, and total cholesterol and LDL decreased, though not significantly, which was on par with the results of Piji H et al., [2]. The HDL raising and LDL, total cholesterol and VLDL decreasing scope of Sitagliptin had been proved by Shimoda S et al., [16]. Present study results were also identical to Shimoda S et al., including a significant rise in HDL levels and an insignificant decrease in other lipid parameters by Sitagliptin monotherapy. The combination regimen showed a favourable lipid profile by decreasing the total cholesterol significantly compared with vehicle and Sitagliptin/Bromocriptine monotherapy. The HDL levels remain unchanged and LDL and VLDL levels were decreased significantly. Though the high dose and normal dose combination showed a more significant change in lipid profile than the low dose combination, the hypoglycaemic effect might limit the use of these combinations (Normal and high doses).
Renal parameters: Sitagliptin and Bromocriptine when given individually, showed no significant change in the renal parameters. But the add-on therapy had a favourable effect in decreasing the urea and creatinine levels significantly (p<0.001) in lower and normal dosage combinations. Sitagliptin had been reported to cause renal damage when given in higher doses [17]. In this study, the high dose combination therapy showed an increase in renal parameters which go in conjunction with the study conducted by Eligar VS and Bain SC [17]. The dosage of Sitagliptin needs to be reduced in renal impairment as demonstrated in the efficacy and safety study of Sitagliptin in chronic renal insufficiency patients [8]. Based on the present study, Bromocriptine, which was shown to be Reno protective [2], could be combined with Sitagliptin (low dose) for decreasing the blood sugar in type 2 diabetic patients with impaired renal function.
Liver parameters: Drug induced liver injury is not frequent with Sitagliptin monotherapy [18]. In this study, the increase in liver enzymes observed with Sitagliptin is to a lesser extent with minimal reversal of AST/ALT ratio (0.9). Bromocriptine was found to cause more liver injury with an AST/ALT ratio of 0.7 which was on par with the report of previous study [19]. The combination regimen showed a decrease in liver injury than Bromocriptine drug given alone. This shows that, the combination therapy has a beneficial effect, by a lesser drug induced damage to liver compared to monotherapy.
Many studies have been done based on the effect of Sitagliptin and Bromocriptine monotherapy in decreasing the fasting blood sugar levels [2,3,7,8,10,11]. No study has been conducted by combining Sitagliptin and Bromocriptine in different doses exploring their synergistic effect in animal models. Standard diabetic animal model was used for this study. Adverse effect on the renal and liver parameters were also monitored and showed as this combination in low doses has exhibited both renal and hepatoprotective activities. A favourable lipid profile was also observed in the rats, which could be helpful in decreasing the mortality among type 2 diabetes mellitus.
Synergism of these drugs shown in this study paves way for an effective alternative for new target identification and drug development which is challenging in treatment of Diabetes Mellitus.
Limitation(s)
Though the study explored the synergistic activity of Sitagliptin + Bromocriptine combination, there are certain limitations and future scopes. The rats were followed-up only for a period of 4 weeks from which the long-term microvascular complications (diabetic neuropathy and retinopathy) could not be monitored. A serial blood sugar monitoring was not done throughout the course of the study, and hence could not explain the potency of the drug combination. The pharmacokinetics of the drug combination is yet to be explored and Insulin assay was not done which might provide a better insight on the drug’s synergism.
Conclusion(s)
It was concluded that the combination therapy is a better treatment regimen compared with the monotherapy of Sitagliptin and Bromocriptine from the results of this standalone study. And of all the treatment combinations, the low dose combination has shown a better glycaemic control, hepatoprotective, hypolipidemic and Reno protective effect. Based on present study, the decrease in body weight due to Bromocriptine was maintained in the combination therapy too. Hence, Sitagliptin and Bromocriptine can be used as an effective treatment for type 2 diabetes mellitus patients, who are refractive to treatment with conventional anti-diabetic drugs. It is worthwhile to consider this aspect for further clinical trials and application in patients with type 2 diabetes mellitus.
*p<0.05, **p<0.01, ***p<0.001 as compared with vehicle group; #p<0.05, ##p<0.01, ###p<0.001 as compared with Sitagliptin group; $p<0.05, $$p<0.01, $$$p<0.001 as compared with Bromocriptine group; Comparison was done by one way-ANOVA followed by Post-Hoc Dunnett’s (2-sided) test; HDL: High density lipoproteins, LDL: Low density lipoproteins, VLDL: Very low density lipoprotein
Values are expressed as Mean±SD for all six groups (n=8 in each group); *p<0.05, **p<0.01, ***p<0.001 as compared with vehicle group; #p<0.05, ##p<0.01, ###p<0.001 as compared with Sitagliptin group; $p<0.05, $$p<0.01, $$$p<0.001 as compared with Bromocriptine group.
Comparison was done by one way-ANOVA followed by Post-Hoc Dunnett’s (2-sided) test; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase