Laboratory Diagnosis of SARS-CoV-2 Infection: Single Centre Experience of First 12,000 Samples
Prashant Patil1, Pratik Thosani2, Santosh Karade3, Kavita Bala Anand4, SPS Shergill5, Sourav Sen6, Rajiv Mohan Gupta7
1 Resident, Department of Microbiology, Armed Forces Medical College, Pune, Maharashtra, India.
2 Resident, Department of Microbiology, Armed Forces Medical College, Pune, Maharashtra, India.
3 Associate Professor, Department of Microbiology, Armed Forces Medical College, Pune, Maharashtra, India.
4 Associate Professor, Department of Microbiology, Armed Forces Medical College, Pune, Maharashtra, India.
5 Associate Professor, Department of Microbiology, Armed Forces Medical College, Pune, Maharashtra, India.
6 Professor and Head, Department of Microbiology, Armed Forces Medical College, Pune, Maharashtra, India.
7 Professor and Dean, Department of Microbiology, Armed Forces Medical College, Pune, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sourav Sen, Professor and Head, Department of Microbiology, Armed Forces Medical College, Pune, Maharashtra, India.
E-mail: sensourav@hotmail.com
Introduction
First spotted in Wuhan, China, World Health Organisation declared the deadly outbreak caused by novel Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) as global pandemic on March 11, 2020. With over 72 million cases globally till December 2020, countries need to gear up to detect, isolate and treat Corona Virus Disease-2019 (COVID-19) cases. Laboratories play a crucial role in diagnosis thereby instituting early contact tracing and quarantine measures. The laboratory based COVID-19 diagnostic testing data may help in formulating strategies to contain the spread of infection.
Aim
To describe key patient variables of the respiratory samples processed for SARS-CoV-2 by Real Time Polymerase Chain Reaction (RT-PCR) at a diagnostic laboratory.
Materials and Methods
In this descriptive, single centre study carried out at ICMR approved COVID-19 diagnostic laboratory, nasopharyngeal swabs received in Viral Transport Medium (VTM) were tested for SARS-CoV-2 infection by RT-PCR. Key patient variables, like age, gender, symptoms and sample positivity rate were tabulated. The demographic and clinical data of samples tested were summarised by medians and Interquartile Range (IQR) for continuous variables and by proportions for categorical variables.
Results
A total of 12,187 samples were received between 21st March to 8th July 2020. The data from 11,196 individuals were complete and were included in the analysis. Overall, 2,053 samples were tested positive for SARS-CoV-2 indicating positivity rate of 18.33%. Sample positivity was highest (63.91%) among high-risk contacts of a laboratory confirmed case. The maximum number of samples tested belonged to age group of 21-40 years and male predominance was observed.
Conclusion
Although social distancing, mask usage, hand hygiene and respiratory etiquettes are important measures for containment of COVID-19, strengthening and capacity building of laboratory network is crucial for mitigating the pandemic.
Acute respiratory syndrome coronavirus-2, Pandemic, Real time polymerase chain reaction, Respiratory infection
Introduction
The year 2020 began with an enormous public health challenge due to emergence and spread of new respiratory viral infection called COVID-19. First detected at the seafood market of the Wuhan city of China, this infectious disease spreads primarily through droplets of upper respiratory tract secretions of an infected person [1]. The disease was declared pandemic by WHO on 11th March 2019 and by 4th April over one million cases of COVID-19 were reported world over [2]. In India, the first case and first death due to COVID-19 was detected on 30th January and 12th March 2020, respectively [3]. After initial resistance, the cases started increasing steadily in India and by 20th December 2020, there were over 10 million cases, the second largest globally [4]. The country rose to the challenge and implemented multiprong surveillance and laboratory testing strategy for containment of COVID-19. The Indian Council for Medical Research (ICMR), New Delhi expanded the COVID-19 diagnostic capacity through existing Viral Research and Diagnostic Laboratory (VRDL) network and capacity building of laboratories of Government institutions, Medical colleges as well as accredited laboratories of private set-ups [5].
By end of August the total number of COVID-19 cases in India, crossed 3.5 million [6]. With around 0.75 million cases, the state of Maharashtra led Indian tally of COVID-19 cases [6]. Two cities from Maharashtra, Pune and Mumbai emerged among top five cities contributing towards maximum number of active cases and maximum number of deaths due to COVID-19. The VRDL network of laboratories strengthened the daily SARS-CoV-2 infection testing capacity across India [5]. Analysis of laboratory level surveillance data will help us to understand the current trend of epidemic and vulnerable population. During COVID-19 diagnostic testing, the laboratory generated valuable data with key variables of interest, namely age, gender, presence or absence of symptoms, co-morbidities, hospitalisation, for characterising current trend of pandemic. These key variables may help in understanding the disease progression and the spread of COVID-19 in the community. The laboratory data would be helpful for program managers in making strategic plans to contain the COVID-19 pandemic. Thus, this was a descriptive study of respiratory samples that were received primarily from Pune and Nashik District of Maharashtra, India tested at this facility.
Materials and Methods
This descriptive study was carried out at Department of Microbiology of a tertiary care teaching hospital over a four month period between 21st March 2020 and 8th July 2020. Nasopharyngeal and/or oropharyngeal (NP/OP) swabs were collected in VTM and were received in a triple layer packaging and maintaining the cold chain from dependent collection centre as per COVID-19 sample collection guidelines given by ICMR [7,8]. As per current ICMR strategy everyone was grouped in to one of nine patient categories based on symptoms and clinical details [8]. Based on symptoms, patients were grouped as asymptomatic, Influenza Like Illness (ILI) and Severe Acute Respiratory Infection (SARI). Patient profile was taken into consideration as Healthcare Worker (HCW) or pregnant women or traveller. History of contact to a confirmed case was also noted. The testing was performed by ICMR approved RT-PCR kit, Super Script (SS) III Invitrogen, for qualitative detection of SARS-CoV-2 genetic targets; Envelope (E gene), Hong Kong University open reading frame 1b (HKU) and Ribonucleic Acid (RNA) dependent RNA Polymerase (RdRP) gene [9]. To begin with, RNA extraction from the NP swab in VTM was performed using spin column based method. Extracted RNA was subjected to RT-PCR as per standard operating procedures of ICMR-NIV (National Institute of Virology) [9]. The patient level data was captured from the specimen referral form submitted by each hospital and from the online ICMR portal (https://cvstatus.icmr.gov.in/). Confidentiality of patients was maintained, and all individuals were identified by unique sample identification number.
Statistical Analysis
The patient level variables were tabulated in Microsoft Excel spreadsheet for further analysis. The variables studied included age, gender, place of residence, symptom status, hospitalisation status. The demographic and clinical data of samples tested were summarised by medians and IQR for continuous variables and by proportions for categorical variables.
Results
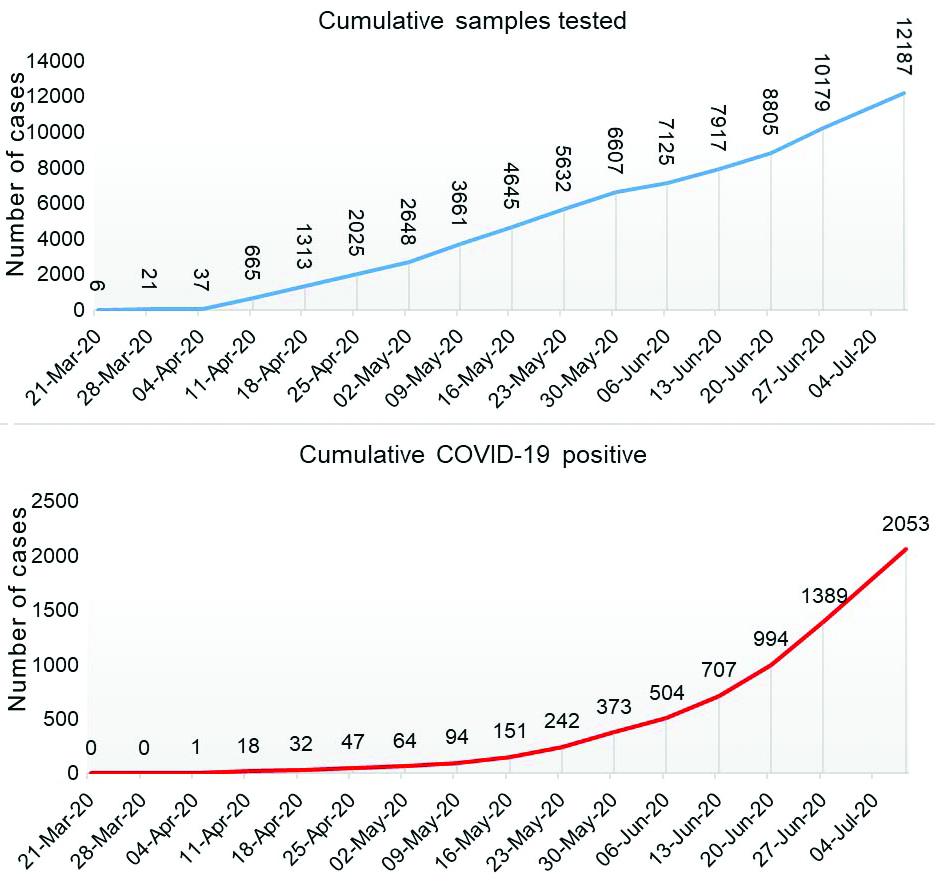
A total of 12,187 samples were received between 21st March to 8th July 2020. However, the data from 11,196 individuals were complete and were included in the analysis. Overall, 2053 samples were tested positive for SARS-CoV-2 indicating positivity rate of 18.33%. A steady increase in the monthly average positivity rate was noted during the study period. The COVID-19 positivity in the month of March, April, May, June, and July were 3.84% (n=26), 2.27% (n=2422), 7.83% (n=4222), 28.50% (n=4070) and 34.96% (n=1447), respectively (date-wise data is given in supplementary file). The daily sample received and the cumulative sample tested along with positivity trend is shown in [Table/Fig-1,2].
SARS-CoV-2 infection trend of respiratory samples tested between 21st March 2020 to 8th July 2020.
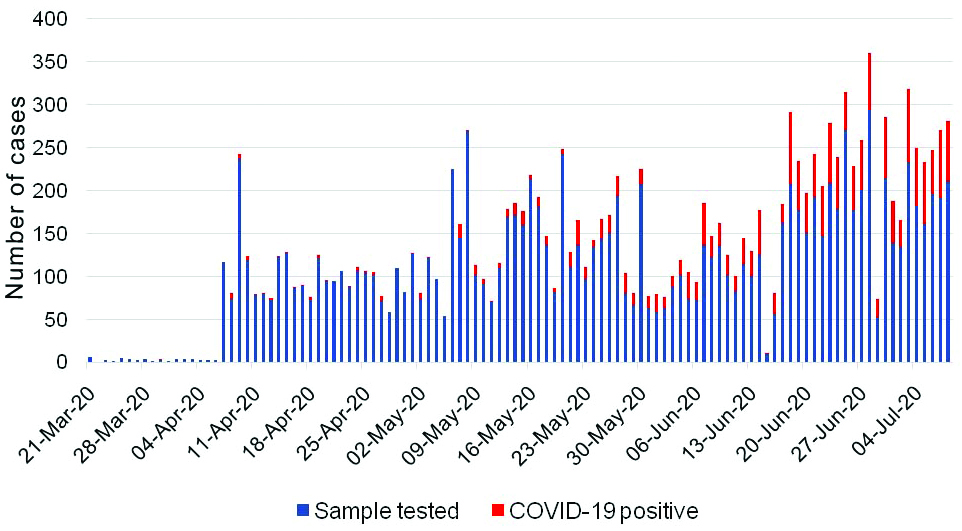
Cumulative sample tested for SARS-CoV-2 infection and COVID-19 positivity-trend from March to July 2020.
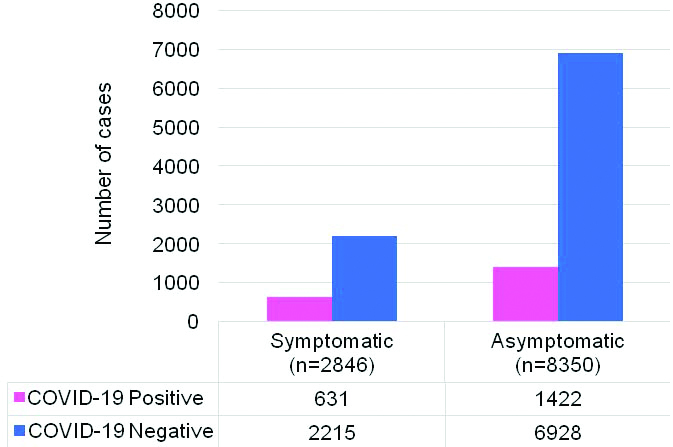
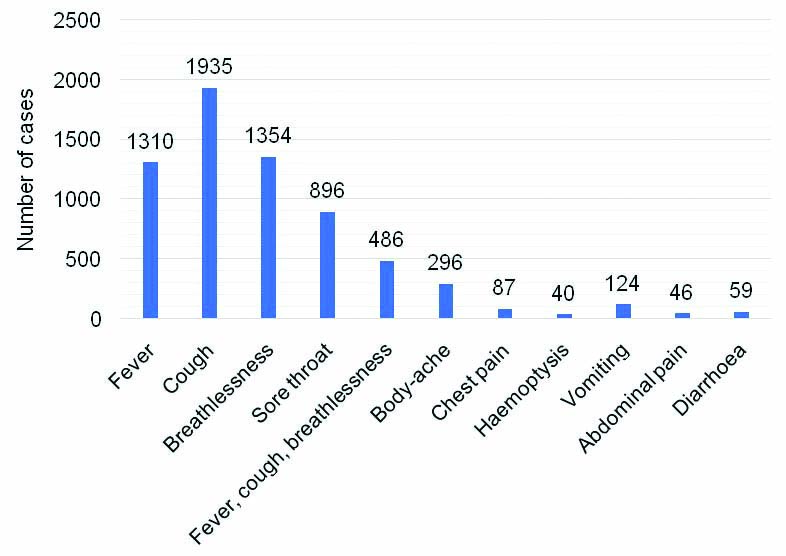
The study population was male predominant (n=6871; 61.37%) with median (IQR) age of 33 (24-46) years. SARS-CoV-2 positivity was highest (27.62%) in individuals above 61 years of age. The positivity rate in each age group is shown in [Table/Fig-3]. Of these 11,196 individuals, 74.58% were found to be asymptomatic. The SARS-CoV-2 positivity rate in individuals with SARI was 14.17%. The SARS-CoV-2 positivity rate among asymptomatic and symptomatic individuals were 17.02% and 22.17%, respectively, and this difference was not significant [Table/Fig-4]. The common symptoms at the time of testing are shown in [Table/Fig-5]. Distribution of patients in various ‘ICMR patient category’ (Category 1 to 9) is shown in [Table/Fig-6].
Age-wise distribution of sample tested for SARS-CoV-2 and COVID-19 positivity-rate.
| Age group (years) | Sample tested | COVID-19 positive | Percentage positivity |
|---|
| <2 | 140 | 27 | 19.29 |
| 2-10 | 749 | 120 | 16.02 |
| 11-20 | 1100 | 244 | 22.18 |
| 21-30 | 2902 | 419 | 14.44 |
| 31-40 | 2618 | 425 | 16.23 |
| 41-50 | 1653 | 323 | 19.54 |
| 51-60 | 1147 | 250 | 21.80 |
| 61 and above | 887 | 245 | 27.62 |
| Total | 11196 | 2053 | 18.33 |
COVID-19 detection in symptomatic versus asymptomatic individuals (n=11,196).
Frequency of symptoms in individuals at the time of SARS-CoV-2 diagnostic testing.
Distribution of patients in ICMR patient category.
| Category | Defining criteria | Number of patients | COVID-19 positive | Percentage positive |
|---|
| 1 | All symptomatic (ILI) individuals with history of International travel in the last 14 days | 96 | 4 | 0.19 |
| 2 | All symptomatic (ILI symptoms) contacts of laboratory confirmed cases | 629 | 128 | 6.23 |
| 3 | All symptomatic (ILI symptoms) health care workers | 295 | 16 | 0.78 |
| 4 | All patients of Severe Acute Respiratory Infection (SARI) | 1002 | 291 | 14.17 |
| 5a | Asymptomatic direct and high risk contact of laboratory confirmed case | 6548 | 1312 | 63.91 |
| 5b | Asymptomatic HCW in contact with confirmed case without protection | 976 | 51 | 2.48 |
| 6 | Symptomatic ILI in hospital | 515 | 142 | 6.92 |
| 7 | Pregnant woman in near labour | 24 | 2 | 0.10 |
| 8 | Symptomatic (ILI) among returnees and migrants (<7 days of illness) | 93 | 8 | 0.39 |
| 9 | Symptomatic ILI in Hotspot/Containment zones | 143 | 47 | 2.29 |
| 10 | Others* | 875 | 52 | 2.54 |
| Total | 11196 | 2053 | 100% |
(Others* category include cases not satisfying criteria for category 1 to 9)
ILI: Influenza like illness; HCW: Health care worker
Highest number of SARS-CoV-2 infected cases were seen in asymptomatic direct and high risk contacts of lab confirmed cases, i.e., ICMR category 5a (63.91%). Health Care Workers (HCW) belonging to category 3 (symptomatic HCW) and 5b (asymptomatic HCW) constituted 11.35% (n=1271) of the sample tested. The overall SARS-CoV-2 positivity rate in symptomatic HCW and asymptomatic HCW were 0.78% and 2.48%, respectively.
Discussion
Globally, COVID-19 pandemic has affected over 232 countries with over 25 million cases till the end of August [10]. India is currently ranked third with over 3.5 million cases [10]. Elevated infectivity of the virus coupled with high population density, sparse resources and reckless human behaviour have fuelled the spread of pandemic in India. However, in a short span of four months, the all round efforts of Government of India were successful in expanding COVID-19 diagnostic testing capacity. By 11th June 2020, there were 877 ICMR approved labs testing for COVID-19 [11]. Working on “Test, Track and Treat” strategy, for early detection and containment of cases, presently there are 1400 ICMR approved laboratories operational across India [11,12]. Currently, testing data across all diagnostic laboratories is entered into unified ICMR data entry portal, as per the ICMR guidelines [5]. This will help in guiding the National strategies appropriately. In this descriptive study, authors have analysed data from over 12,000 individuals tested at the laboratory from March to July 2020. Maharashtra is leading in number of COVID-19 cases in India. In this study, the overall SARS-CoV-2 sample positivity rate was 18.33%. Present study data indicate increase in SARS-CoV-2 positivity from 3.03-28.42% from March to July 2020.
The highest number of samples was received from age group of 21-40 years with an average positivity rate of 15.33%. Data from other cities of Maharashtra and rest of India also indicate maximum burden of COVID-19 in younger population [5,12]. People of 21-40 years age groups constitute working class and are less compliant to interventions like quarantine, public use of mask and social distancing. Overcrowding in urban cities of Pune and Nashik at home, workplace or during commuting, could explain higher positivity in younger population. Younger people being less compliant had increased risk of getting exposed to infection. Besides many young people being asymptomatic carriers were the main drivers of the pandemic. Also, the data may be skewed towards younger age group as younger people are more approachable to COVID-19 testing. Whereas, older people are restricted to home and are subjected to testing only when they get sick. This study indicated highest (27.62%) SARS-CoV-2 positivity in individuals above 61 years of age. Prior studies have also indicated higher affection and mortality in elderly individuals [13,14]. This age group have additional co-morbidity in making them prone for increased severity of disease and hospitalisation. Existing COVID-19 statistics also indicate higher mortality rate in Maharashtra (3.5%) as compared to the rest of India (2.05%) [15].
Results indicate male predominance which corroborates with other studies [16,17]. Males are more susceptible to SARS-CoV-2 infection due to higher expression of ACE-2 receptors in males as compared to females [16]. Besides females are found to have higher macrophage and neutrophil activity with higher antibody production [16]. Moreover, in Indian setting, males contribute to a higher percentage of outdoor working population thereby increasing the risk of exposure.
Although the number of asymptomatic people tested was higher, there was no difference in the COVID-19 positivity rate between symptomatic and asymptomatic people. The asymptomatic individuals are main source of spread of infection in susceptible Indian population. The higher asymptomatic population is indicative of active contact tracing efforts. The commonest symptoms reported were fever, cough and breathlessness. The study results indicated considerable affection of HCW. These HCW are the real COVID-19 warriors, involved in day-to-day management of infected patients and their personal health needs to be safe guarded. The HCW should be on priority list of immunisation, whenever a protective vaccine is made available.
Limitation(s)
The study results should be seen in the light of certain limitations. The study analysed the samples received only during the initial four months of pandemic. Non-pharmacological intervention and government policy will influence the future course of pandemic in Indian setting. Also, authors could not ascertain the outcome of the patient following detection of SARS-CoV-2 infection.
Conclusion(s)
This descriptive study is preliminary assessment of 11,196 samples tested for SARS-CoV-2 by RT-PCR at our laboratory from March to July 2020. The study showed rising positivity trend of COVID-19 requiring strengthening and capacity building of laboratory network for early diagnosis. The disease containment strategy needs to be focussed on younger population. Integration of laboratory would provide nationwide data for generating meaningful strategies.
(Others* category include cases not satisfying criteria for category 1 to 9)
ILI: Influenza like illness; HCW: Health care worker
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? No
Was informed consent obtained from the subjects involved in the study? No
For any images presented appropriate consent has been obtained from the subjects. No
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Dec 16, 2020
Manual Googling: Feb 15, 2021
iThenticate Software: Mar 23, 2021 (7%)
[1]. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, A novel coronavirus from patients with pneumonia in China, 2019N Engl J Med 2020 382:727-33.10.1056/NEJMoa200101731978945 [Google Scholar] [CrossRef] [PubMed]
[2]. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 29 June 2020. Source [Internet]. Available at https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---29-june-2020 [Google Scholar]
[3]. Ministry of Health and Family Welfare, Government of India. MoHFW. COVID-19 Resources. Source [Internet]. Available at from https://www.mohfw.gov.in Last assessed on Aug 15, 2020 [Google Scholar]
[4]. World Health Organization. Weekly Epidemiological Update on COVID-19. Source [Internet]. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update---12-january-2021 [Google Scholar]
[5]. ICMR COVID Study Group, COVID Epidemiology & Data Management Team, COVID Laboratory Team, VRDLN TeamLaboratory surveillance for SARS-CoV-2 in India: Performance of testing & descriptive epidemiology of detected COVID-19, January 22 - April 30, 2020Indian J Med Res 2020 151:424-37.10.4103/ijmr.IJMR_1896_203261191432242875 [Google Scholar] [CrossRef] [PubMed] [PubMed]
[6]. World Health Organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
[7]. National Centre for Disease Control, New Delhi. Specimen Collection, Packaging and Transport Guidelines for 2019 nCoV-Acute Respiratory Disease. Source Internet. Available at: https://ncdc.gov.in/WriteReadData/l892s/50471431021580628750.pdf [Google Scholar]
[8]. Indian Council of Medical Research, New Delhi. Strategy for COVID-19 testing in India (Version 5, dated 18/05/2020). [Source: Internet]. Available at: https://www.icmr.gov.in/cteststrat.html [Google Scholar]
[9]. ICMR National Institute of Virology, Pune. Standard operating procedure for detection of 2019 nCoV by rRT-PCR. ICMR-NIV; 2020. Pp.1-6. [Source: Internet] https://www.icmr.gov.in/pdf/covid/labs/2_SOP_for_Confirmatory_Assay_for_2019_nCoV.pdf [Google Scholar]
[10]. Worldometer 2020. COVID-19 coronavirus pandemic. [Source: Internet]. Available at: https://www.worldometers.info/coronavirus/. Last assessed on Aug 15, 2020 [Google Scholar]
[11]. Indian Council of Medical Research, New Delhi. COVID-19 dashboard; list of COVID-19 testing labs. Available at https://www.icmr.gov.in/ [Google Scholar]
[12]. Gupta N, Potdar V, Praharaj I, Giri S, Sapkal G, Yadav P, Laboratory preparedness for SARS-CoV-2 testing in India: Harnessing a network of Virus Research & Diagnostic LaboratoriesIndian J Med Res 2020 151(2 & 3):216-25. [Google Scholar]
[13]. Munayco C, Chowell G, Tariq A, Undurraga EA, Mizumoto K, Risk of death by age and gender from COVID-19 in Peru, March-May, 2020Aging (Albany NY) 2020 12(14):13869-81.10.18632/aging.10368732692724 [Google Scholar] [CrossRef] [PubMed]
[14]. Li X, Xu S, Yu M, Wang K, Tao Y, Risk factors for severity and mortality in adult COVID-19 inpatients in WuhanJ Allergy Clin Immunol 2020 146(1):110-18.10.1016/j.jaci.2020.04.00632294485 [Google Scholar] [CrossRef] [PubMed]
[15]. COVID-19 INDIA. (2020). COVID-19 India Dashboard. Available at: https://www.covid19india.org [Google Scholar]
[16]. Kopel J, Perisetti A, Roghani A, Aziz M, Gajendran M, Goyal H, Racial and gender-based differences in COVID-19Front Public Heal 2020 8:01-08.10.3389/fpubh.2020.0041832850607 [Google Scholar] [CrossRef] [PubMed]
[17]. Klein SL, Morgan R, The impact of sex and gender on immunotherapy outcomesBiol Sex Differ 2020 11(1):01-13.10.1186/s13293-020-00301-y32366281 [Google Scholar] [CrossRef] [PubMed]