The VZV which causes varicella in children and HZ in elderly is a DNA virus belonging to herpes group of viruses. Varicella infection occurs in childhood and more than 85% of the population is affected before the age of 15 years [1]. VZV persists in the latent form after causing varicella and gets reactivated in the later years causing HZ. HZ is characterised by the occurrence of grouped vesicles on an erythematous base [2] which generally involves the entire dermatome innervated by a single spinal or cranial sensory ganglion [3] and is also associated with radicular pain. Lesions are usually associated with neuralgic symptoms which may be pre-eruptive or postherpetic. Occasionally, asymptomatic lesions may occur [4] or dermatomal pain/burning sensation around the lesions (Zoster sine herpes) [5] may be seen. The disease has a self-limiting course in immunocompetent individuals; however extensive and atypical presentations such as multi dermatomal involvement or disseminated distribution of lesions are seen in the immunocompromised [6].
Materials and Methods
A three year longitudinal cohort study of 212 cases suffering from HZ was carried out in Department of Dermatology during period of February 2017 to January 2020. Ethical approval was obtained from Institutional Ethics Committee.
Inclusion criteria: All adults in the age group of 18 to 60 years, clinically diagnosed with HZ (multiple grouped vesicles on erythematous base in dermatomal distribution) were included.
Exclusion criteria: All cases who presented after 72 hours of onset of symptoms, those who had already received antiviral therapy and those with poor general condition (i.e., unstable vital signs in an acutely ill patient) were excluded.
Informed consent was obtained from all the included cases and clinical photographs were taken. The clinical history and findings were recorded in a prestructured proforma (containing details regarding history, co-morbidities, clinical examination and dermatomal involvement). History of immunosuppression (diabetes mellitus, tuberculosis, chemotherapy and HIV drugs) was elicited. Out of 267 total cases, 55 cases were lost to follow-up. Investigations like serum HIV antibody test (ELISA) and Tzanck smear examination were done in all cases. All diagnosed cases of HZ were treated with Oral Acyclovir 800 mg five times a day for seven days [12], topical application of Fusidic acid 2% cream twice a day until healing of lesions and Oral Diclofenac 50 mg and Paracetamol 500 mg twice daily after meals for seven days. Cases were advised to come for follow-up every fortnightly for six weeks. During follow-up, cases were evaluated for relief of symptoms, treatment outcome and complications/sequelae like PHN (persistence or recurrence of pain more than a month after onset of HZ) [13] and paraesthesia, pigmentation, scarring and ulceration.
Statistical Analysis
It was done using software Statistical Package for the Social Sciences (SPSS) version 20.0. Results were expressed in frequency and percentage.
Results
Out of 212 cases, 123 (58.01%) were males and 89 (41.98%) were females. Majority, 83 (39.15%) belonged to 41-50 years of age. [Table/Fig-1].
Age and sex wise distribution of cases (N=212).
| Age group (years) | Males | Females | Total n (%) |
|---|
| 18-30 | 12 | 14 | 26 (12.26%) |
| 31-40 | 35 | 24 | 59 (27.83%) |
| 41-50 | 47 | 36 | 83 (39.15%) |
| 51-60 | 29 | 15 | 44 (20.76%) |
| Total | 123 (58.01%) | 89 (41.98%) | 212 (100%) |
Most common presenting symptoms were skin lesions in association with burning pain observed in 118 (55.66%) cases and itching in 74 (34.90%) cases. Twenty (9.43%) cases were asymptomatic. Almost all cases n=191 (90%) had symptoms 1-2 days before onset of skin lesions. History of prodromal illness like fever, headache and arthralgia was present in 40 (18.86%) cases. Rest 172 cases (81.13%) had no prodromal symptoms.
Out of 212 cases, 149 (70.29%) had no co-morbidities, while 63 (29.71%) had co-morbidities, like diabetes mellitus in 31 (49.20%), autoimmune diseases like pemphigus vulgaris in 3 (4.76%), systemic lupus erythematosus in 6 (9.52%), rheumatoid arthritis in 3 (4.76%) and inflammatory bowel disease in 4 (6.34%) cases. All these cases were on immunosuppresive therapy [Table/Fig-2].
Co-morbidities in cases of HZ (n=63/212).
| Co-morbities | No. of cases n (%) |
|---|
| Diabetes mellitus | 31 (49.20%) |
| Acquired immunodeficiency syndrome | 08 (12.69%) |
| Chemotherapy for malignancy and lymphomas | 05 (07.93%) |
| Pemphigus vulgaris | 03 (4.76%) |
| Systemic lupus erythematosus | 06 (9.52%) |
| Rheumatoid arthritis | 03 (4.76%) |
| Inflammatory bowel disease | 04 (6.34%) |
| Eruptive lichen planus | 01 (1.58%) |
| Psoriasis and psoriatic arthritis | 02 (3.17%) |
| Total | 63 |
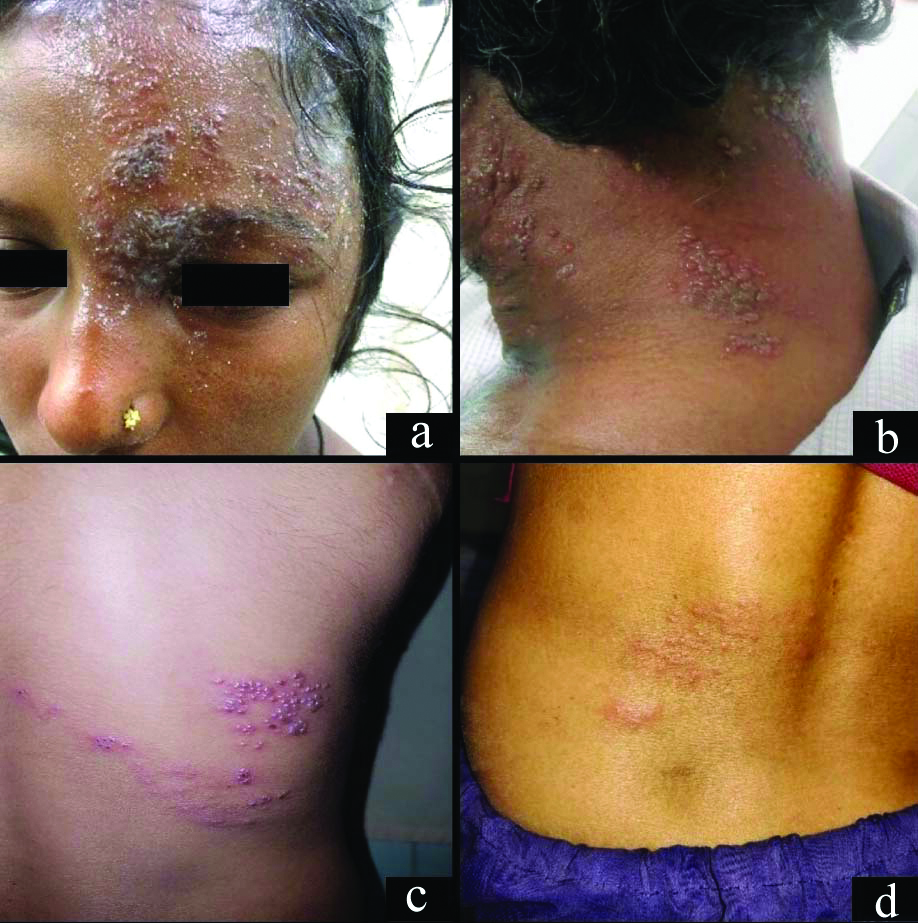
The distribution of lesions of HZ along various dermatomes is given in [Table/Fig-3]. Out of 47 cases having cranial nerve involvement, 40 cases had trigeminal nerve (ophthalmic, maxillary and mandibular division in 22, 9 and 9 cases, respectively) [Table/Fig-4a] and seven cases had facial nerve involvement. Ramsay Hunt syndrome was observed in two out of seven cases having facial nerve involvement. Cervical [Table/Fig-4b] and thoracic dermatomes [Table/Fig-4c] were most commonly involved (27.35% each) followed by cranial (22.16%), lumbar (17.92%) [Table/Fig-4d] and sacral (5.18%) dermatomes [Table/Fig-3].
Dermatome wise distribution of lesions of Herpes Zoster (HZ) (n=212).
| Nerves | Male (right/left) | Female (right/left) | Total (%) |
|---|
| Cranial | 28 (18/10) | 19 (13/6) | 47 (22.17%) |
| Cervical | 31 (23/8) | 27 (23/4) | 58 (27.36%) |
| Thoracic | 37 (25/12) | 21 (13/8) | 58 (27.36%) |
| Lumbar | 19 (10/9) | 19 (7/12) | 38 (17.92%) |
| Sacral | 08 (5/3) | 03 (2/1) | 11 (05.19%) |
| Total | 123 | 89 | 212 (100%) |
a) HZ involving ophthalmic division of trigeminal nerve; b) HZ involving cervical region; c) HZ involving thoracic region; d) HZ lesions over lumbar region.
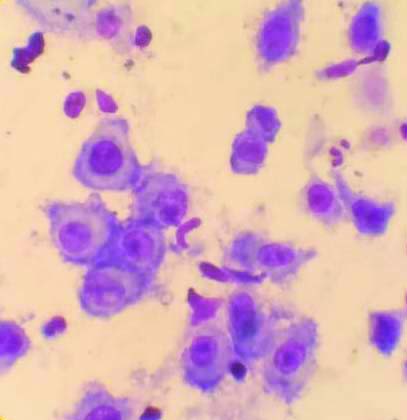
Tzanck smear was examined for multinucleated giant cells in all 212 cases and it was positive in 172 cases [Table/Fig-5]. Eight cases tested positive for serum HIV antibodies. They were referred to ART centre for further management.
Tzanck smear showing Multinucleated giant cells on 40X magnification using Giemsa stain.
Oral Acyclovir was well tolerated by 74 (34.91%), while 81 (38.21%) cases had nausea and vomiting, 42 (19.81%) developed headache and 15 (7.07%) had diarrhoea [Table/Fig-6].
Adverse Drug Reactions (ADR) due to Oral Acyclovir (n=212).
| ADR | Number of cases n (%) |
|---|
| Nausea and vomiting | 81 (38.21%) |
| Headache | 42 (19.81%) |
| Diarrhoea | 15 (07.08%) |
| Well tolerated (no ADR) | 74 (34.90%) |
| Total | 212 (100%) |
During follow-up, the details regarding healing of lesions, symptoms, treatment outcome and complications/sequelae like PHN and paraesthesia (n=80, 37.73%) [Table/Fig-7], more in patients in their 5th and 6th decades of life was observed. Pigmentation (n=54, 25.47%), scarring (n=31, 14.62%) [Table/Fig-8] and ulceration with secondary infection (n=15,7.07%) were noted [Table/Fig-9]. Motor involvement like facial palsy (Ramsay Hunt syndrome) and neurological complications like tranverse myelitis (involving sensory, motor and autonomic nervous systems) were seen in two cases (0.94%) and one case (0.47%) each respectively.
PHN in various age groups.
| Age (years) | Number of cases of HZ | Number of cases of PHN |
|---|
| Male | Female | Total |
|---|
| 18-30 | 26 | 5 | 3 | 08 (30.76%) |
| 31-40 | 59 | 12 | 9 | 21 (35.59%) |
| 41-50 | 83 | 19 | 14 | 33 (39.75%) |
| 51-60 | 44 | 11 | 7 | 18 (40.90%) |
| Total | 212 | 47 | 33 | 80 |
Depicting scarring due to HZ.
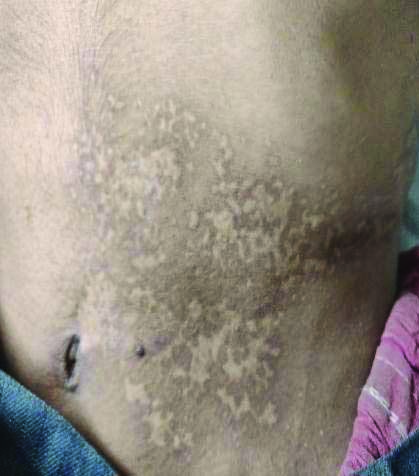
Complications/sequalae of HZ (n=212).
| Complications | Number of cases n (%) |
|---|
| Postherpetic neuralgia and paraesthesia | 80 (37.73%) |
| Pigmentation | 54 (25.47%) |
| Scarring | 31 (14.62%) |
| Ulceration with secondary infection | 15 (07.07%) |
| Ramsay hunt syndrome | 2 (0.94%) |
| HZM | 01 (00.47%) |
| No complications | 29 (13.67%) |
| Total | 212 |
Discussion
In the present study the majority of patients belonged to 5th (39.15%) decade. The male:female ratio was 1.4:1. Co-morbidities were seen in 29.71% cases. Thoracic dermatome and cranial nerve involvement were seen in equal number of cases followed by the cervical segment. As per the study by Abdul Latheef EN and Pavithran K, the highest number of cases were in 4th decade (55%) and males outnumbered females in the ratio of 1.3:1 [14]. Co-morbidities were present in 30% cases. Thoracic segment was commonly involved, followed by cranial nerve involvement in their study [Table/Fig-10]. Further, in a study by Babamahmoodi F et al, in North Iran male:female ratio was 1.5:1. Co-morbidities were present in 40% cases. Cranial nerve involvement was commonly seen followed by involvement of thoracic nerves [15].
Comparative data of Abdul Latheef EN and Pavithran K with the present study.
| Parameters | Study by Abdul Latheef EN and Pavithran K [14] | Present study |
|---|
| Age (decade) | 4th | 5th |
| Sex | 1.3:1 | 1.4:1 |
| Co-morbidities | 30% | 29.71% |
| Dermatome involved | Thoracic>cranial | Thoracic=cranial >cervical |
| Presenting symptom | Pain and skin lesions | Pain and skin lesions |
In this study, an increased incidence of HZ was seen in cases on chemotherapy (7.93%). In a study done by Insinga RP et al., 8.7% (n=865) were on chemotherapy [16] while, in a study by Kim ST et al., it was found that this incidence was same as compared to healthy patients [17]. An increased incidence of HZ was seen in cases with diabetes mellitus (14.62%), while in a study by Heymann AD et al., 8.5% cases had diabetes mellitus [18]. As per the study by Gauthier A et al., the incidence of PHN was 19.5% (after one month of onset of rash) and 13.7% (after three months of onset of rash), while in the present study PHN was seen in 37.73% cases after one month of onset of rash [19]. Antiviral medication might have some consequence on the severity of acute pain and the duration of skin lesions [20]. Oral antiviral medication may shorten the duration of PHN [21].
In this study, eight patients (12.69%) were HIV positive, out of which three patients were on Anti-retroviral therapy since 2-3 months and five were newly detected HIV positive (one patient each of disseminated HZ and multidermatomal zoster and three patients of HZ ophthalmicus). All were started on Acyclovir 800 mg five times a day and showed delayed healing after seven days of treatment. One patient of HZ ophthalmicus went on to develop Ramsay Hunt syndrome. Compared to normal individuals, incidence of HZ is six times more common in HIV positive patients [22]. VZV reactivation was found in 3% of HIV seropositive patients compared with 0.2% of HIV seronegative patients. Nadir CD4 counts below 200 cells/μL and detectable viremia are reported as the two major risk factors for HZ occurrence in HIV positive patients [11]. There is no recommendation for use of topical antiviral agents in treatment of HZ [23].
Acyclovir when started within 72 hours of onset of HZ, results in quick recovery of skin lesions and decreases incidence of PHN. It should be started even in patients with comorbidities. Patients on antiretroviral therapy may show delayed healing of lesions inspite of starting Acyclovir and have a higher risk of complications.
Limitation(s)
The impact of varicella vaccination in HZ and its complications have not been studied. Patients on oral Acyclovir who presented to the skin OPD beyond 72 hours were not included in the study. This is a descriptive study and the outcome with respect to age and gender was not included in the study.
Conclusion(s)
Herpes Zoster is usually diagnosed based on clinical appearance of vesicular skin lesions associated with burning pain in a dermatomal distribution. Individuals in fourth and fifth decades of life are commonly affected. Cervical and thoracic dermatomes are commonly involved. Antiviral therapy with Acyclovir is recommended for patients of all age groups. It has been observed that acyclovir shortens viral shedding, stops the progression of disease and reduces neuralgic pain. The best time to start Acyclovir is within 72 hours of symptoms. It is likely to reduce the impact of acute phase of HZ on the quality of life. Acyclovir also reduces neural damage that may occur before onset of rash. It is well tolerated and safe. Systemic antiviral therapy (Acyclovir) has a favourable balance of benefit vs risk and is strongly recommended as first line treatment for all HZ patients.