Introduction
Endodontic infection requires the successful removal of microorganisms from the root canal system. The most effective irrigant solution is sodium hypochlorite, but possible problems due to its toxicity require the look for new alternatives. Constant increases in antibiotic resistance and side-effects caused by chemical irrigation have shifted research towards the production of herbal alternatives, especially in paediatric dentistry.
Aim
To evaluate and compare the antibacterial efficacy of miswak, green tea, chamomile and 0.5% NaOCl as an endodontic irrigants against Enterococcus faecalis in primary root canals.
Materials and Methods
The in-vitro study was conducted at the Department of Conservative Dentistry and Department of Microbiology, College of Dentistry, University of Duhok from January 2020 till June 2020. Seventy five freshly extracted intact human mandibular primary molars were decoronated at Cemento-Enamel Junction (CEJ) and distal roots were separated and biomechanically prepared up to F3 Protaper file and stored in normal saline. The specimens were inoculated with Enterococccus faecalis suspension and incubated for 72 hours. Specimens were divided into five groups containing fifteen teeth each (n=15). Freshly prepared alcoholic extracts of miswak, green tea and chamomile were used as an irrigant solution against E.faecalis compared to NaOCl as positive control and normal saline as negative control. Swabs were collected using F3 protaper paper points. The number of colonies was counted in suitable plate under good illumination and manual lens for magnification. Statistical analysis was performed by using Kruskal-Wallis one-way Analysis and Student-Newman-Keuls Method. Statistical Package for the Social Sciences (SPSS) version 26.0 was used. The p-values more than 0.05 were considered as statistically non significant.
Results
Green Tea, among the herbal experimental groups, had the most effective antibacterial effect against E.faecalis. No significant statistical difference was detected between green tea (p=0.272) and NaOCl; however, there was significant difference between miswak, chamomile and NaOCl as well as between the rest herbal experimental groups and normal saline.
Conclusion
NaOCl remains the gold standard as irrigant in primary teeth. Green tea extract may help in reducing E.faecalis inside the canals of primary teeth. Good efficacy against E.faecalis was also shown by Miswak and chamomile however the results obtained were not significant when compared with NaOCl.
Introduction
Primary teeth are as important as permanent teeth for the harmonious growth of occlusion, preservation of arch length, chewing and speech [1]. Endodontic treatment helps preserve the function of excessively carious primary teeth. Appropriate root canal therapy depends on the combination of good instrumentation, irrigation and root canal obturation. Intracanal irrigants can enhance mechanical debridement by flushing out waste, dissolving tissues and disinfecting the root canal system [2].
Irrigants play an important role in paediatric endodontics due to inconsistent internal structure and characteristics such as internal connections and lateral anastomoses seen in primary teeth that are rare in permanent teeth [3]. Microorganisms found in the asymptomatic root canal differ from those found in the clinically symptomatic root canal. E.faecalis, Porphyromonasgingivalis and Treponemadenticola have been found to be the most prevalent bacterial species in infected root canals of primary teeth [4]. In primary root canal infections, the highest portion of the microorganisms is detected deeper in the lateral canals, apical implications and dentinal tubules [5].
The most effective irrigant is sodium hypochlorite (NaOCl) which has excellent tissue dissolving capacity and antimicrobial properties, but also has some undesirable characteristics, such as tissue toxicity, risk of emphysema when overfilled, allergic propensity, unpleasant odour and taste, inability to remove the smear layer and the mutagenic potential of parachloroanaline product when combined with chlorhexidine (CHX) [6].
The use of herbal products in therapeutic goods has been used since ancient times and has increased considerably over the last few decades [7]. Due to the disadvantages of most common intracanal drugs used, such as cytotoxicity, and their failure to extract bacteria from dental tubules, the trend of recent medicines to use biologic medicines derived from natural plants is growing. The main benefits of using herbal alternatives are: easy availability, cost-effectiveness, improved shelf life, low toxicity and observed lack of microbial resistance [5].
Green tea polyphenols, a popular drink from Japan and China, are made from young leaves of a tea plant called (Camellia sinensis). Statistically, green tea polyphenols exhibited significant antibacterial activity against E.faecalis biofilm formed on the tooth substrate [8].
For decades, German chamomile (Marticariarecutitia L.) has been used as a medicinal plant primarily for its anti-inflammatory, analgesic, antimicrobial, antispasmodic and sedative properties [9]. After comparing the chamomile extract and tea tree oil to 2.5% NaOCl for smear layer removal, they concluded that chamomile has the ability to remove the smear layer better than NaOCl, but lesser than the combination of NaOCl and Ethylenediamine Tetraacetic Acid (EDTA). The smear layer removal could be attributed to the presence of acidic components (capric acid, caprylic acid, chlorogenic acid, o-caumaric acid, p-caumaricacid, dihydroxybenzoic acid) in the extract [10]. So far, few studies have discussed the antibacterial effect of chamomile as irrigant solution.
Miswak is derived from the plant Salvadora persica, primarily used as a chewing stick, which is used for the cleansing of teeth. By isolating the active ingredient from S. Persica, it was noticed that limonoid has a strong antimicrobial capacity by inhibiting the growth of various Gram-positive and Gram-negative microorganisms by preventing extra polysaccharides and glycosidase enzyme produced by the microorganism [11].
Studies, about the effect of herbal extract (green tea, miswak and chamomile) used as root canal irrigants in primary teeth, have not been found. Hence, this study was conducted to assess the antibacterial efficacy of indigenously prepared herbal extracts and compare it with 0.5% NaOCl endodontic irrigant solution for canals infected with E.faecalis in primary teeth. Without sacrificing its antimicrobial effectiveness, it is essential to use the least cytotoxic irrigation solutions during endodontic therapy.
Materials and Methods
This was an in-vitro study conducted at the Department of Conservative Dentistry and Department of Microbiology, College of Dentistry/University of Duhok, Iraq, from January 2020 till June 2020.
The method of herbal extraction was as follows: 200 gm of Miswak (S.persica) was cut into irregular pieces and dried in an oven at a temperature of 45°C±5°C for a period of 24 hours until it became completely moisture free and then was grounded by an electric miller (high speed multi-functional crusher) to form a fine powder [12]. A 200 gm of green tea leaves and German Chamomile were also separately grounded into powder form. Then, 150 gm of each herbal powder was soaked in 300 mL of 96% ethanol inside a flask and kept at room temperature [13,14]. After 72 hours, the solutions were filtered using a filter paper. The ethanol was evaporated and the extracts were concentrated using a rotary flask evaporator. These extracts were stored at 4°C in refrigerator until need [14].
Exclusion and Inclusion criteria: A total of 75 extracted human mandibular of first and second primary molars were collected from the polyclinic dental center and a private clinic and were used for this study. For the purpose of this study, primary molars, with atleast 2/3rd remaining root, which were extracted due to the presence of chronic abscess and/or sinus tract were chosen. Any tooth with root caries, more than two-thirds loss of root structure or a patient with antibiotic use for systemic diseases in the past 4 weeks were excluded from the study.
Herbal extraction: Teeth debris and soft tissue remnants was cleansed using ultrasonic scaling device (ACTEON, Newtron, France). The anatomical crown of all the teeth were amputated at CEJ perpendicular to the long axis of the teeth by a water cooled diamond disc bur (Tompet, Norderstedt, Germany) and the roots were separated. Only the distal roots were used in the study. The exploration of the radicular canal was accomplished with size 15-K file (NIC, England, United Kingdom). The working length of roots measured (10±1) mm. All teeth were immersed in normal saline until required [15].
Protaper system rotary files were used to prepare the root canal biomechanically to size F3 (30/9%) (Dentsply-Maillefer, Ballaigues, Switzerland). The canals were irrigated with 0.5% NaOCl (CERKAMED, Poland) for disinfection of the canals and final rinse with normal saline was done [14].
The apical foramen of each root sample was sealed by applying flowable composite (Harvard, GmbH, Germany) on the root apex to prevent fluid leakage during microbiological study. Each root was embedded in silicon impression material block-up and 2 mm of cervical margin was exposed to facilitate the grasping and holding of root samples [Table/Fig-1]. The specimens were wrapped with aluminum foil and then autoclaved (mocom, Italy) at 121°C for 30 minutes [16].
Samples embedded in silicone impression material.

The samples were divided randomly into five groups of 15 teeth for each. In Group 1, 0.5% NaOCl was used as a positive control root canal irrigant. While in Group 2, 0.9% normal saline was used as negative control. Group 3, 4 and 5 were irrigated with miswak extract, green tea extract and chamomile, respectively.
The standard strain of E.faecalis (ATCC®29212™), obtained from Media Laboratory Private (Hawler/Iraq), was prepared by harvesting (4-5) colonies with a previously sterilized circular loop and dissolved into a glass container containing 3 mL Brain-Heart Infusion (BHI) broth. The cultures were grown overnight at 37°C in the BHI broth and the growth was determined by change in turbidity after 24 hours [17]. A volume of 10 μL of suspension was inoculated into each tooth and incubated for 72 hours at 37°C to allow growth of E.faecalis inside the root canals. The specimens were handled using flamed tweezers to prevent contamination [18].
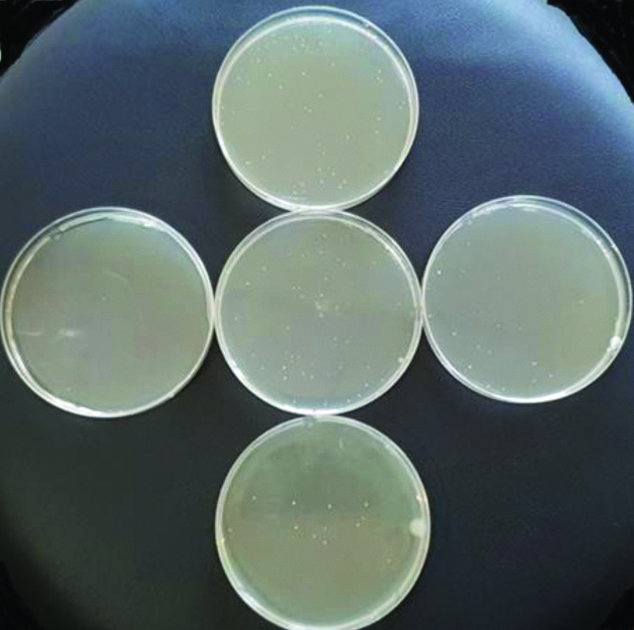
Canals were filled with 2 mL of the corresponding irrigant in each group and were permitted to stay for 10 minutes. The root canals were then rinsed with 2 mL of saline for 1 minute. Sterile paper point (size F3) (DIADDENT, Korea) was left in the wet canal for 1 minute and was transferred into a test tube containing 1 mL sterile normal saline for the first tube and 0.8 mL normal saline for the other 5 tubes to form (1-6) tubes for the serial dilution. After mixing well 0.2 mL of tube number 1 had been transferred to tube number 2 and the same procedure was done for the another tubes. From this 1 mL of each dilution 100 μL was pipetted onto a sterile Mueller-Hinton agar plate. These plates were incubated for 24 hours at 37°C in an incubator. After incubation, the colonies were counted under good illumination by using camera zoom (mobile phone telescope, 8X, China) [Table/Fig-2]. Then the number of the colonies was multiplied by the dilution factor to get Colony Forming Unit (CFU) [19].
Culture plates show the colonies of bacteria on a Mueller-Hinton agar.
Sensitivity test: A 0.1 mL of BHI broth of E.faecalis was cultured on Mueller-Hinton agar plates. Wells of 5 mm diameter were made in the agar surface. Then tested materials were added to these wells. After 24 hours of incubation at 37°C, the diameter of inhibition zones were measured [20].
Statistical Analysis
The comparison of colony counts among study groups was examined by using Kruskal-Wallis one-way Analysis. Statistical Package for the Social Science (SPSS) version 26.0 was used. Student-Newman-Keuls Method used for the comparison of colony counts between positive and negative controls and other study groups. A p-value less than 0.05 were considered significant.
Results
The total number of CFU/mL obtained from the experimental groups after 72 hours of incubation is presented in [Table/Fig-3] and comparison between study groups is presented in [Table/Fig-4]. Antibacterial activity of green tea extract was most effective against E.faecalis among the herbal experimental groups followed by miswak and chamomile respectively. No statistically significant difference (p>0.272) was found between the green tea extracts and between NaOCl. NaOCl showed maximum antibacterial efficacy against E.faecalis, while the antibacterial property of 0.9% normal saline had the least effect against E.faecalis. The results of inhibition zone of sensitivity test are presented in [Table/Fig-5].
Numbers of Colony Forming Units (CFU) of E.faecalis for all study groups.
| GroupsPlates No. | NaOCl | Normal Saline | Miswak | Green Tea | Chamomile |
|---|
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 240×102 | 140×102 | 0 | 0 |
| 5 | 0 | 250×102 | 188×102 | 0 | 0 |
| 6 | 0 | 260×102 | 32×102 | 0 | 0 |
| 7 | 0 | 224×102 | 60×102 | 0 | 39×102 |
| 8 | 0 | 53×102 | 27×102 | 0 | 195×102 |
| 9 | 0 | 41×102 | 24×102 | 0 | 250×102 |
| 10 | 0 | 175×102 | 40×102 | 0 | 38×102 |
| 11 | 0 | 210×102 | 32×102 | 28×102 | 37×102 |
| 12 | 0 | 250×102 | 96×102 | 25×102 | 80×102 |
| 13 | 26×102 | 180×102 | 74×102 | 34×102 | 65×102 |
| 14 | 28×102 | 170×102 | 100×102 | 26×102 | 190×102 |
| 15 | 25×102 | 155×102 | 110×102 | 28×102 | 180×102 |
| Total | 79×102 | 1974×102 | 932×102 | 141×102 | 1074×102 |
Student-Newman-Keuls test comparing the number of CFU in each group.
| Comparison | Diff. of Ranks | p<0.05 (Significant Differences) |
|---|
| NS vs NaOCl | 510.000 | <0.001 |
| NS vs Green Tea | 457.000 | <0.001 |
| NS vs Chamomile | 205.500 | 0.012 |
| NS vs Miswak | 162.500 | <0.001 |
| NaOCl vs Green Tea | 53.000 | 0.272 |
| NaOCl vs Miswak | 347.500 | 0.002 |
| NaOCl vs Chamomile | 304.500 | <0.001 |
| Green Tea vs Miswak | 294.500 | <0.001 |
| Green Tea vs Chamomile | 251.500 | <0.001 |
| Miswak vs Chamomile | 43.000 | 0.373 |
ns: Normal saline; NaOCl: sodium hypochlorite
Size of inhibition zone for all experimental groups in mm.
| Groups | Inhibition Zone in mm |
|---|
| NaOCl | 10 |
| Normal Saline | 0 |
| Miswak | 7 |
| Green Tea | 12.5 |
| Chamomile | 8 |
Discussion
Primary teeth are essential in the life of the child as they help chew, speak, contribute to esthetics, maintain the integrity of the dental arches and finally lead the teeth to their correct position [5]. In early childhood, carious teeth are not only predictive of potential dental problems, but can adversely affect development and cognitive growth by affecting nutrition [21].
Effective endodontic treatment of primary teeth relies on the eradication (if present) of microbes from the root canal region and the prevention of re-infection [5]. The root canal treatment is done under continuous irrigation with hand or rotary instrumentation to extract the inflamed and necrotic tissues, microbes/biofilms and other root-canal space debris [22]. Due to its high prevalence in secondary endodontic infections, E.faecalis was selected for this study [23].
Optimal irrigation is based on the usage of a mixture of two or more irrigants in a particular series to meet the aim of safe and efficient irrigation [22]. Despite its unpleasant odour and taste for the child and the possible risks of swallowing and poisoning during the procedure, the most commonly used irrigant solution is NaOCl, which also has burning properties and lacks differentiation between necrotic and vital tissues when in contact with apical and periapical tissues [24]. It also has adverse effects on dentin, such as lowering its flexural strength and elastic modulus. NaOCl toxicity to vital periodontal tissues, risk of emphysema when overfilling, allergic potential had been demonstrated [14]. Risk of damage to permanent tooth follicles, peripheral tissue and oral mucosa, through inappropriate use of NaOCl has been noted in paediatric endodontic patients [24].
Common used concentration of NaOCl range from 0.5-5.25%, so to reduce the cytotoxicity of NaOCl especially in paediatric dentistry, 0.5% NaOCl was used as positive control in this study. Herbal extracts were used to disinfect the root canal in primary teeth to overcome the undesired effects of NaOCl. Herbal medicinal products gain importance due to their therapeutic properties and biocompatibility [9]. The present study evaluates antibacterial efficacy of herbal irrigants in comparison with 0.5% NaOCl which is commonly used in concentrations ranging from 0.5% to 5.25%. Hypochlorite is reported to destroy the target microorganisms within seconds even at low concentrations [25].
Studies have compared the biological effects of mild and strong NaOCl solutions and demonstrated greater cytotoxicity and caustic effects on healthy tissue with 5.25% NaOCl than with 0.5% and 1% solutions [2]. The relationship between the concentration and cytotoxicity of NaOCl had been reported by Chang YC et al., 2001. Therefore, it is recommended to replace 5.25% of NaOCl by 0.5-1% NaOCl for canal irrigation [26]. For this study, 0.5% NaOCl tested as positive control. NaOCl showed highest antibacterial effect (79×102 CFU) [Table/Fig-3] against E. faecalis compared to herbal extracts, while normal saline had the least (1974×102 CFU) [Table/Fig-3].
In the present study, authors found that green tea showed a good antibacterial activity against E. faecalis compared to the control group with (141×102 CFU) [Table/Fig-3]. Green tea has active physiological materials that have great curing, antioxidant action, anti-inflammatory, important scavenging properties that are all helpful to make it ideal for intracanal irrigation. Other good features of green tea, as a cleaning agent, include accessibility, affordability, low toxicity and long shelf life [27].
The current study agrees with Ramezanali F et al., who demonstrated that green tea showed acceptable antibacterial effect on the biofilm of E. faecalis [14]. Nonsignificant results were obtained when CFU in green tea and NaOCl group were compared (p>0.0001). Similarly, the results of this study are compatible with the American Medical Association which showed that green tea has excellent medicinal values. It is also observed that green tea has antibacterial effect against E.faecalis [28]. Also, Jerin J et al., showed that 2.5% NaOCl had the best antimicrobial property against E. faecalis, followed in descending order by green tea extract, garlic extract and neem leaf extract [29].
Miswak (S. persica) showed antibacterial effect against E.faecalis with colony count (932×102CFU) [Table/Fig-3]. The antimicrobial and cleaning effects of Miswak are attributed to different chemicals that can be detected in its extracts. Other effects are thought to be attributable to the presence ofcyanogenic glycoside and benzyl-isothiocyanate and to its high content of NaCl and KCl, as well as Salvadourea and Salvadorine, saponins, tannins, vitamin C, silica, certain naturally occurred anionic components and resin [30].
But results of this study disagreed with Al-Azzawi A, that used agar diffusion methods to compare between antibacterial effect of S.persica and green tea against E.faecalis. The study showed S.persica more effective against E.faecalis than green tea [20]. German Chamomile (Marticariarecutitia L.) extract also showed antibacterial effect against E.faecalis with number of colony (1074×102 CFU) [Table/Fig-3] as a root canal irrigation in primary teeth. A few studies discussed the antibacterial effect of chamomile as intracanal irrigation.
Vakil N et al., observed that chamomile oil exhibited antibacterial activity against gram-positive bacteria such as Bacillus subtilis, Staphylococcus aureus, Streptococcus mutans, and Streptococcus salivarius at a concentration of 25 mg/mL, but at 150 mg/mL concentration, chamomile exhibited no microbial effect against E. faecalis and C. albicans. However, chamomile efficacy is not more than 2% Chlorhexidine, antimicrobial effect against both microorganism had been reported at higher concentration 250 mg/mL [31].
One particular component of the irrigant solution could be attributed to the anti-inflammatory effect. In the German chamomile irrigant solution salicylic acid in the form of a methyl ester provides an anti-inflammatory effect [32]. Flavonoids, including apigenine, chamazulene and α-bisabolol are the other constituents found in whole plant chamomile extract. The flavone act as anti-inflammatory agent which can significantly affect bacteria [33].
In the present study, there was no significant difference statistically between NaOCl and green tea. NaOCl shows the highest antibacterial effect against E.faecalis followed by green tea, miswak and chamomile respectively. Some differences between the results of other studies and our results might be due to the differences in the method of extract preparation, the quality of (green tea, Miswak and Chamomile) and culture time.
Limitation(s)
Ample options to choose specific types of herbs were not available. Only herbs of local origin were obtainable. The applied methods of preservation of herbal materials before being used in this study could be questionable. For sensitivity test, both disc and wells should have been used.
Conclusion(s)
Herbal irrigant solutions showed acceptable antibacterial effect against E. faecalis in primary teeth. Green tea specifically showed no significant difference with NaOCl, whereas significant differences were observed with miswak and chamomile. To avoid undesirable properties of NaOCl, the use of herbal substitutes as intracanal irrigant solutions, particularly in paediatric dentistry, should be considered.
ns: Normal saline; NaOCl: sodium hypochlorite
[1]. Fabbri G, Cannistraro G, Pulcini C, Sorrentino R, The full-mouth mock-up: A dynamic diagnostic approach (DDA) to test function and esthetics in complex rehabilitations with increased vertical dimension of occlusionInt J Esthet Dent 2018 13(4):460-74. [Google Scholar]
[2]. Kandaswamy D, Venkateshbabu N, Root canal irrigantsJ Conserv Dent 2010 13(4):25610.4103/0972-0707.7337821217955 [Google Scholar] [CrossRef] [PubMed]
[3]. Jaju S, Jaju PP, Newer root canal irrigants in horizon: A reviewInt J Dent 2011 2011:85135910.1155/2011/85135922190936 [Google Scholar] [CrossRef] [PubMed]
[4]. Cogulu D, Uzel A, Oncag O, Eronat C, PCR-based identification of selected pathogens associated with endodontic infections in deciduous and permanent teethOral Surg, Oral Medic, Oral Patho, Oral Radio Endo 2008 106(3):443-49.10.1016/j.tripleo.2008.03.00418547832 [Google Scholar] [CrossRef] [PubMed]
[5]. Kashyap N, Irrigating solutions in pediatric dentistry: A big deal in little teethEC Dent Sci 2019 18:1620-26. [Google Scholar]
[6]. Jena A, Sahoo SK, Govind S, Root canal irrigants: A review of their interactions, benefits, and limitationsCompend Contin Educ Dent 2015 36(4):61-256. [Google Scholar]
[7]. Silva FB, Almeida JM, Sousa SM, Natural medicaments in endodontics- A comparative study of the anti-inflammatory actionBraz Oral Res 2004 18:174-79.10.1590/S1806-8324200400020001515311323 [Google Scholar] [CrossRef] [PubMed]
[8]. Prabhakar J, Senthilkumar M, Priya MS, Mahalakshmi K, Sehgal PK, Sukumaran VG, Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and Green tea polyphenols), MTAD and 5% sodium hypochloride against Enterococcus faecalis biofilm formed on tooth substrate: An invitro studyJ Endod 2010 36:83-86.10.1016/j.joen.2009.09.04020003940 [Google Scholar] [CrossRef] [PubMed]
[9]. Venkateshbabu N, Anand S, Abarajithan M, Sheriff SO, Jacob PS, Sonia N, Natural therapeutic options in endodontics-A reviewOpen Dent J 2016 10(1):214-26.10.2174/187421060161001021427386007 [Google Scholar] [CrossRef] [PubMed]
[10]. Lahijani MS, Kateb HR, Heady R, The effect of German chamomile (Marticariarecutitia L.) extract and tea tree (Melaleucaalternifolia L.) oil used as irrigants on removal of smear layer: A scanning electron microscopy studyInt Endod J 2006 39:190-95.10.1111/j.1365-2591.2006.01073.x16507072 [Google Scholar] [CrossRef] [PubMed]
[11]. Halawany HS, A review on miswak (Salvadorapersica) and its effect on various aspects of oral healthSaudi Dent J 2012 24(2):63-69.10.1016/j.sdentj.2011.12.00423960531 [Google Scholar] [CrossRef] [PubMed]
[12]. Al Qarni FM, ElFasakhany FM, Kenawi LM, Moustafa AM, Antimicrobial activity of Azadirachtaindica (neem) and Salvadorapersica (miswak) extracts as endodontic irrigantsEndodontic Practice Today 2019 13(3):237-45. [Google Scholar]
[13]. Pourabbas R, Delazar A, The effect of German chamomile mouthwash on dental plaque and gingival inflammationIran J Pharm Sci 2010 20(2):105-09. [Google Scholar]
[14]. Ramezanali F, Samimi Sh, Kharazifard MJ, Afkhami F, The in vitro antibacterial efficacy of persian green tea extract as an intracanal irrigant on enterococcus faecalis biofilmIran Endod J 2016 11(4):304-08.10.22037/iej.2016.9 [Google Scholar] [CrossRef]
[15]. Thomas S, Asokan S, John B, Priya G, Kumar S, Comparsion of antimicrobial efficacy of diode laser, triphala and sodium hypochlorite in primary root canals: A randomized controlled trialInt J Clin Pediatr Dent 2017 10(1):14-17.10.5005/jp-journals-10005-139928377648 [Google Scholar] [CrossRef] [PubMed]
[16]. Zakarea NA, Mohamad TH, Taqa AA, Chumbley S, Al-Juaid S, Balto H, A newly prepared solution for the removal of the smear layerJ Dent Sci 2014 2(1):19-26.10.12691/ijdsr-2-1-6 [Google Scholar] [CrossRef]
[17]. Mathew J, Sonia P, Jojo K, Ranjith K, Mathew A, Joy M, Evaluation of an indigenously prepared herbal extract (EndoPam) as an antimicrobial endodontic irrigant: An ex vivo studyJ Inter Oral Heal 2015 7(6):88-91. [Google Scholar]
[18]. Kumar H, An in vitro evaluation of the antimicrobial efficacy of Curcuma longa, Tachyspermumammi, chlorhexidine gluconate, and calcium hydroxide on Enterococcus faecalisJ Conserv Dent 2013 16(2):144-47.10.4103/0972-0707.10819723716967 [Google Scholar] [CrossRef] [PubMed]
[19]. Suresh P, Rhitu Sh, Rohit P, Manoj H, Amit G, A comparative evaluation and effectiveness of different antimicrobial herbal extracts as endodontic irrigants against Enterococcus faecalis and Candida albicans an in vitro studyUniver J Dent Scie 2018 4:2 [Google Scholar]
[20]. Al-Azzawi A, The antibacterial effect of herbal alternative, green tea and Salvadorapersica (siwak) extracts on Enterococcus faecalisJ Bagh Coll Dentistry 2015 27(2):01-05.10.12816/0015287 [Google Scholar] [CrossRef]
[21]. King NM, Anthonappa RP, Itthagarun A, The importance of primary dentition to children part 1: Consequence of not treating carious teethHK Pract 2007 29:52-61. [Google Scholar]
[22]. Haapasalo M, Shen Y, Wang Z, Gao Y, Irrigation in endodonticsBritish Dent J 2014 216(6):299-303.10.1038/sj.bdj.2014.20424651335 [Google Scholar] [CrossRef] [PubMed]
[23]. Alghamdi F, Shakir M, The influence of enterococcus faecalis as a dental root canal pathogen on endodontic treatment: A systematic reviewCureus 2020 12(3):e725710.7759/cureus.7257 [Google Scholar] [CrossRef]
[24]. Chaugule VB, Panse AM, Gawali PN, Adverse reaction of sodium hypochlorite during endodontic treatment of primary teethInt J Clin Pediatr Dent 2015 8(2):153-56.10.5005/jp-journals-10005-130426379387 [Google Scholar] [CrossRef] [PubMed]
[25]. Kaur R, Singh R, Sethi K, Garg S, Miglani S, Irrigating solutions in pediatric dentistry: literature review and updateJ Adv Med Dent Scie 2014 2(2):104-15. [Google Scholar]
[26]. Chang YC, Huang FM, Tai KW, Chou MY, The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cellsOral Surg Oral Med Oral Pathol Oral Radiol Endod 2001 92:446-50.10.1067/moe.2001.11681211598582 [Google Scholar] [CrossRef] [PubMed]
[27]. Sinha DJ, Sinha AA, Natural medicaments in dentistryAYU 2014 35(2):113-18.10.4103/0974-8520.14619825558153 [Google Scholar] [CrossRef] [PubMed]
[28]. Anurag S, Anuraag G, Chandrawati G, Comparison of antimicrobial efficacy of conventional irrigants and herbal products alone and with calcium hydroxide against Enterococcus faecalisGuident 2012 5(2):77 [Google Scholar]
[29]. Jerin J, Shoba K, Shibu A, Nithya T, Sheena C, Comparative evaluation of antimicrobial activity of green tea extract, garlic extract, neem leaf extract and sodium hypochlorite as root canal irrigants against E. faecalis and C. albicans-An in vitro studyInt J Curr Microbiol Appl Sci 2015 4:384-91. [Google Scholar]
[30]. Shingare P, Chaugule V, Comparative evaluation of antimicrobial activity of miswak, propolis, sodium hypochlorite and saline as root canal irrigants by microbial culturing and quantification in chronically exposed primary teethGERMS 2011 1(1):12-21.10.11599/germs.2012.100424432254 [Google Scholar] [CrossRef] [PubMed]
[31]. Vakil N, Singh A, Jad B, Kaur B, Evaluation of the antimicrobial activity of different concentrations of chamomile extract and chlorhexidine gel against Candida albicans and Enterococcus faecalisInt J Appl Dent Sci 2019 5(2):218-20. [Google Scholar]
[32]. Pistorius A, Willershausen B, Steinmeier EM, Kreisler M, Efficacy of subgingival irrigation using herbal extracts on gingival inflammationJ. Periodontal 2003 74:616-22.10.1902/jop.2003.74.5.61612816293 [Google Scholar] [CrossRef] [PubMed]
[33]. Hadly S, Petry J, Medicine herbs: a primer for primary careHosp Pract 1999 34:109-12.10.3810/hp.1999.06.15110386114 [Google Scholar] [CrossRef] [PubMed]