Concomitant Tuberculosis (TB) and Hodgkin’s Lymphoma (HL) is a rare entity, more commonly reported in the paediatric and adolescent populations. HL presents with fever, weight loss and lymphadenopathy. Disseminated TB also presents with fever, weight and appetite loss and generalised or localised lymphadenopathy with/without pulmonary symptoms. Both diseases together pose a diagnostic challenge for the clinicians because of the similar mode of presentation. This case report discusses the clinical scenario of a 48-year-old lady presenting with generalised lymphadenopathy and was initially diagnosed with disseminated TB. Due to clinical worsening despite regular Anti-TB Therapy (ATT), she was re-evaluated and later turned out to have co-existing HL.
Case Report
A 48-year-old lady with no prior co-morbidities was presented to the emergency department with the chief complaints of fever for six months which was low grade, intermittent, weight loss for six months (weight-50 kg) associated with loss of appetite, easy fatigability and breathlessness on exertion for three months. There was no history of any habit or addiction. There was no history of orthopnea, Paroxysmal Nocturnal Dyspnea (PND), chest pain, cough with expectoration, Gastrointestinal (GI) or urinary symptoms.
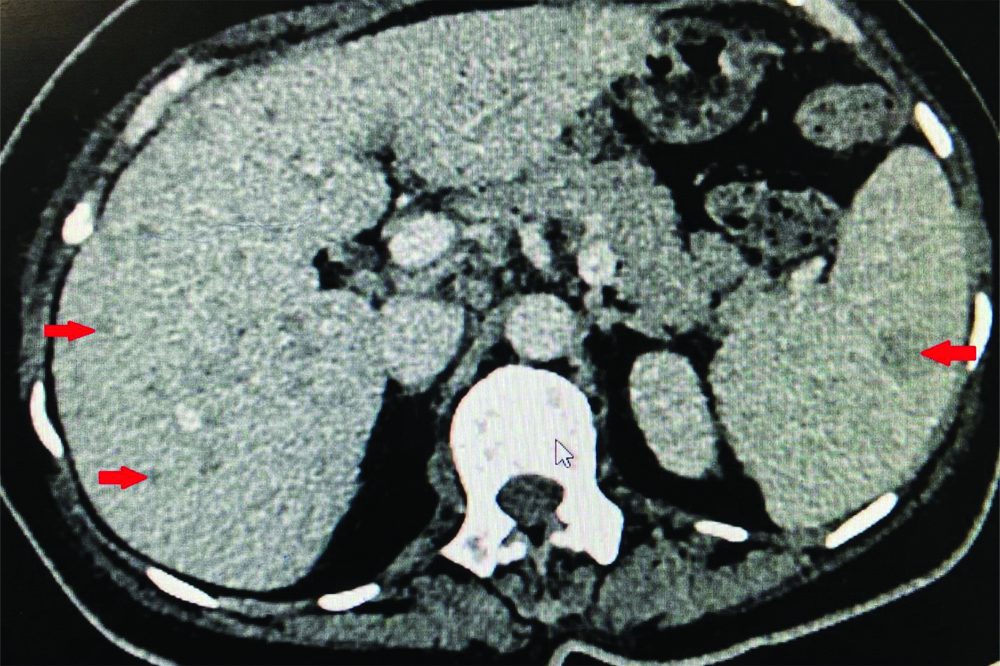
On physical examination, pallor was present, liver was palpable 5 cm below the right costal margin with a span of 16 cm and splenic tip was palpable. Liver and renal function tests were normal, haemogram showed a total leucocyte count of 11,600/μL {Differential count-neutrophils (74%), lymphocytes (6%), eosinophils (15%), monocytes (1%)} and an absolute eosinophil count of 1740/cubic millimeters (cu.mm); haemoglobin (Hb)-5.5 g/dL; platelets-1.9 lac/cu.mm. Peripheral smear showed a normocytic normochromic anaemia with no haemoparasites or atypical cells. Malarial and filarial antigens, Mantoux test (<5 mm), rk 39, blood and urine cultures were negative. Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) serologies were also negative. For the evaluation of eosinophilia, stool for ova and cysts, fungal cultures, serum galactomannan, c-Antineutrophilic Cytoplasmic Antibodies (ANCA), p-ANCA was sent and was negative. Serum Immunoglobulin E levels were normal. Two-Dimensional echocardiography was normal. Ultrasonography (USG) abdomen showed hepatosplenomegaly with multiple enlarged lymph nodes in the periportal, para-aortic, mesenteric and right iliac regions and large conglomerated lymph nodes in the left iliac fossa. Contrast Enhanced Computed Tomography (CECT) chest and abdomen showed calcified nodules and atelectasis in the right upper lobe, sub-centimetric lymph nodes in the pre and paratracheal largest node measuring 1.08 cm and hilar regions; multiple lymph nodes in periportal, para-aortic, mesenteric region largest node measuring 10.2×6.1×8.1 cm with pulled up caecum and inflammatory granulomas in the liver and spleen [Table/Fig-1,2 and 3]. Bone marrow examination was done to rule out malignancy which showed epitheloid granulomas, langhans type giant cells and focal areas of coagulative necrosis [Table/Fig-4]. TB-Polymerase Chain Reaction (PCR) of the bone marrow came out to be positive.
CECT abdomen showing inflammatory granulomas in liver and spleen.
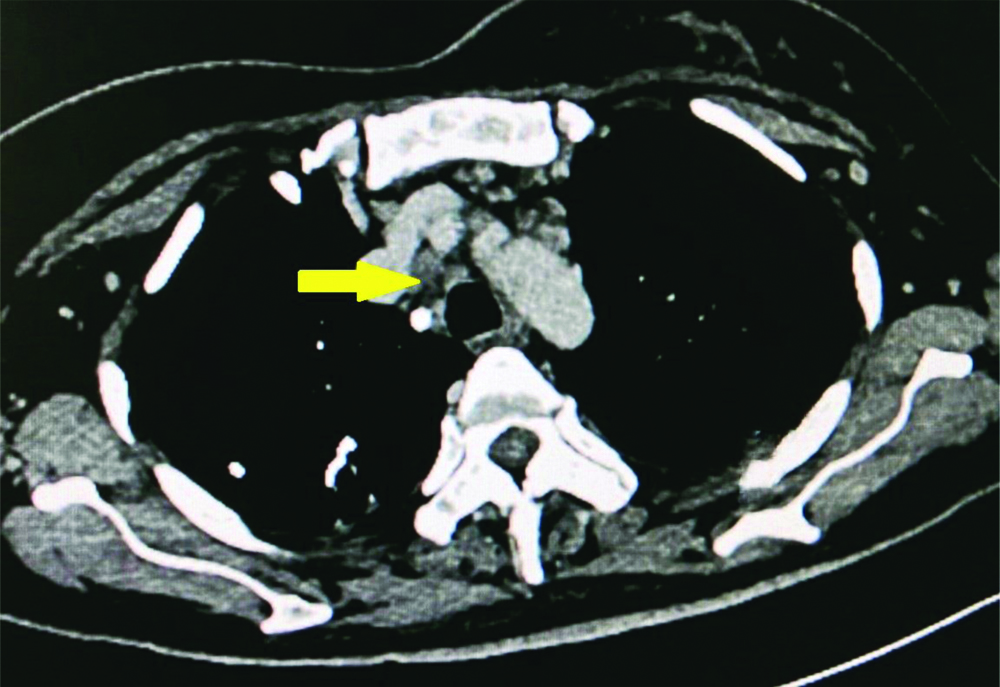
CECT chest showing pretracheal and paratracheal lymphadenopathy.
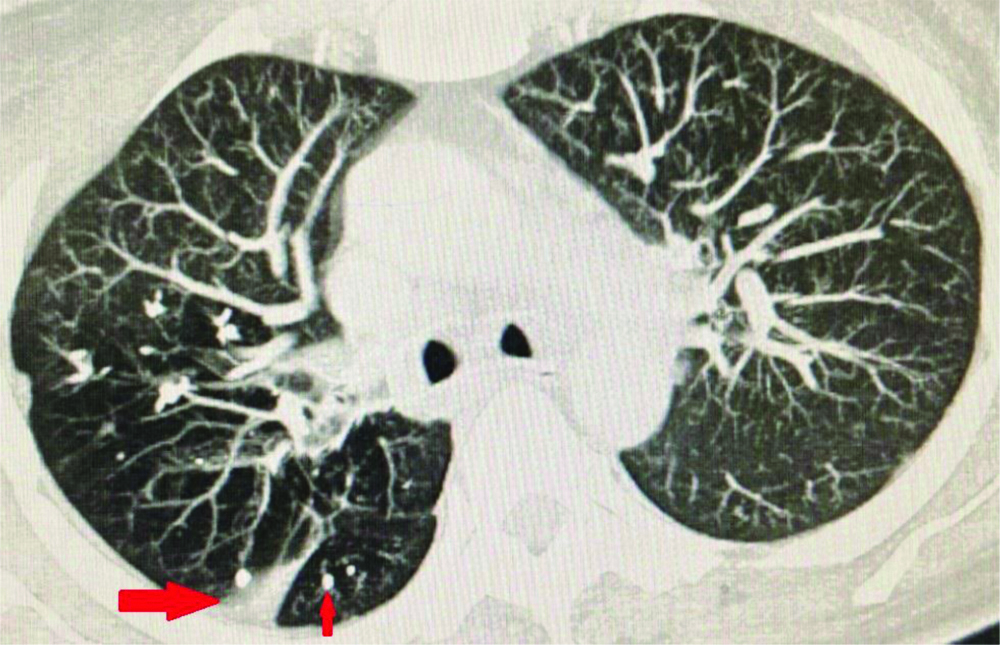
CECT chest showing band of atelectasis and calcified granulomas in right upper lobe.
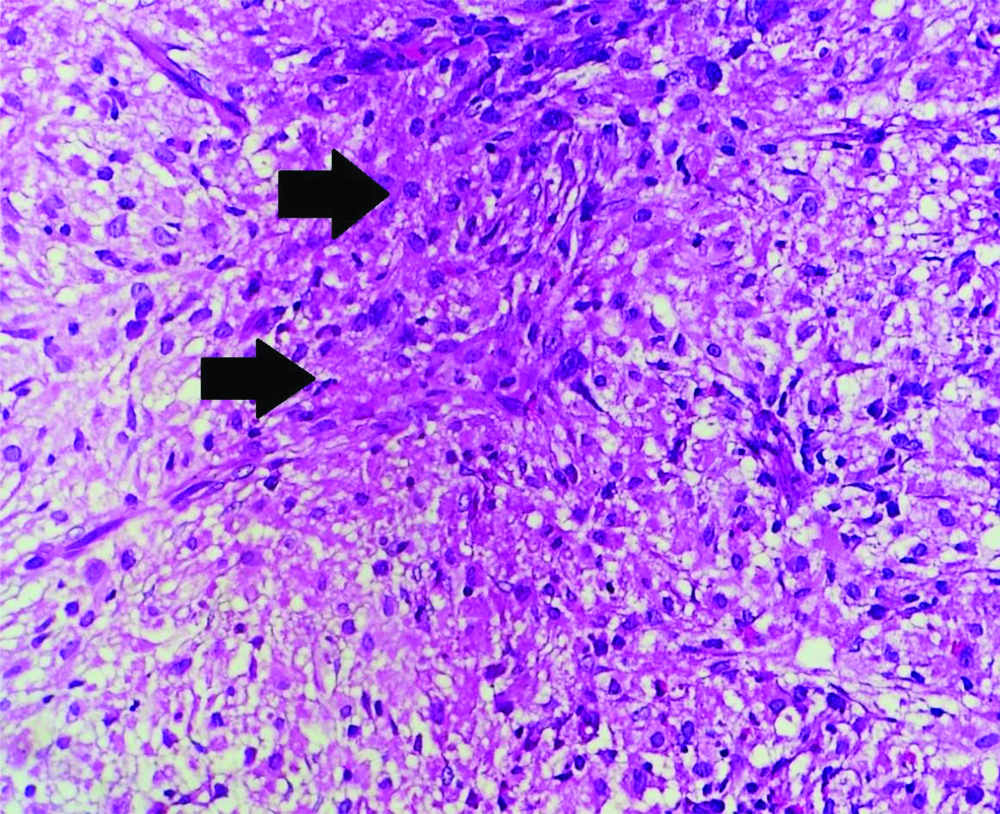
H&E-stained section of bone marrow at 20X showing granuloma and focal areas of coagulative necrosis.
A diagnosis of disseminated TB was made and the patient was discharged on category 1 ATT (Isoniazid-5 mg/kg, Rifampicin-10 mg/kg, Pyrazinamide-25 mg/kg, Ethambutol-15 mg/kg- HRZE) according to weight with advice to follow-up. Patient presented to the emergency department after 10 days with persistent fever and a lump in the inguinal region. Treatment compliance was ensured.
Examination and investigations showed inguinal lymphadenopathy (3×3 cm) and persistent eosinophilia (absolute eosinophil count-5660/cu.mm). Other granulomatous aetiologies namely sarcoidosis and systemic fungal infections were considered and ruled out as serum Angiotensin Converting Enzyme (ACE) levels were normal, fungal cultures and urinary antigen for histoplasma was negative. To rule out resistant TB, inguinal lymph node biopsy was done but TB gene Xpert didn’t show rifampicin resistance. Instead, the biopsy showed the presence of Reed Sternberg (RS) cells [Table/Fig-5]- CD15 negative, CD 30 and PAX 5 positive in RS cells suggestive of mixed cellularity HL. A repeat bone marrow examination was also done, which confirmed the presence of co-existent HL with granulomatous pathology.
H&E stained section of lymph node at 400X showing Reed Sternberg cells (RS); Inset at 400X showing Immunohistochemistry (IHC) of lymph node golgi zone with CD 30 positive RS cells.
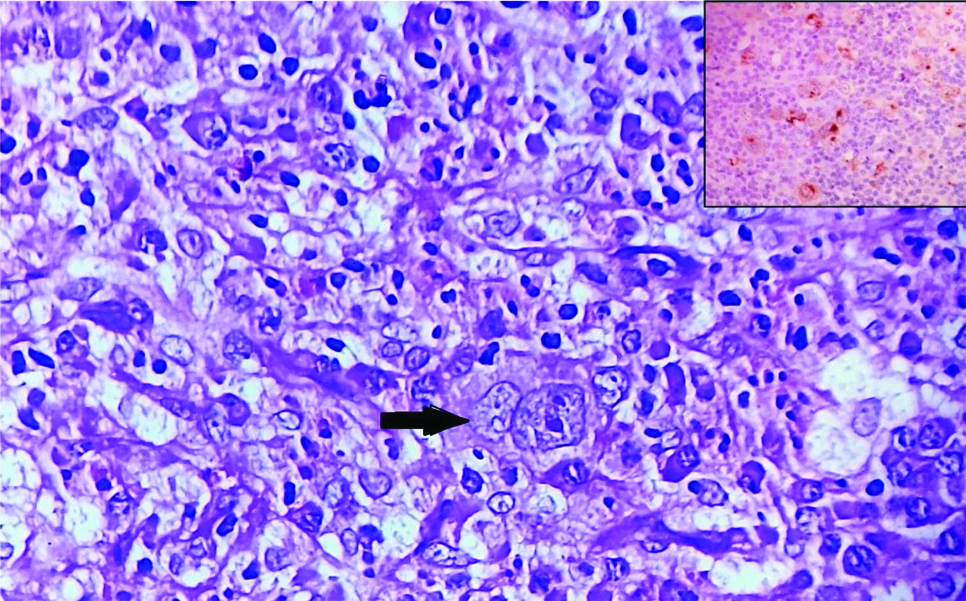
A final diagnosis of disseminated TB with concomitant HL was made. Patient was advised to continue ATT and referred to Oncology department for the management of HL.
Discussion
Hodgkin’s Lymphoma (HL) is a malignancy of mature B lymphocytes and represents 10% of all lymphomas diagnosed each year. Almost 95% cases of HL are nodular sclerosis and mixed cellularity types [1]. Patients present with the TB symptoms (fever, night sweats and/or weight loss) and palpable lymphadenopathy. TB can also present with similar complaints, masquerading as lymphoma. It creates confusion and delay in the diagnosis as it is more common than HL and is the first differential diagnosis of chronic lymphadenopathy in developing countries like India [2].
Immune response in Hodgkin’s disease is predominantly mediated by CD4 (Cluster of Differentiation) cells in involved organ [3]. The pathogenesis of TB infection is mediated by cell-mediated immunity and (Interferon) IFNγ+CD4+T cells and factors such as inherited or acquired states of immunodeficiency, immunosuppressive therapy or malignancy, may facilitate the infection to spread and cause symptomatic diseases [4].
Research has shown direct DNA damage, apoptosis inhibition with Mycobacterium tuberculosis infection favours new vessels and tumours formation. Some of the mycobacterial cell wall components are hypothesised to trigger reactive oxygen species and nitric oxide generation thereby causing DNA damage- a condition termed as inflammation-related carcinogenesis [5,6]. Thus, in patients with concurring TB and HL, whether TB is the primary condition predisposing to HL or the vice versa is debatable as in this case. HL is bimodal in presentation, most commonly presenting among young ages (in 20’s) and in the elderly (in 80’s) with equal male and female distribution [1]. The case described has an unusual age of presentation of Hodgkin’s at 48 years in a female, probably because of the co-existing TB. TB accompanying malignant lymphomas is often characterised by an atypical clinical course, with unusual extrapulmonary localisations [7]. This includes lymph nodes, breast, spleen, liver, jejunum and skin. This patient also presented with generalised lymphadenopathy with liver, spleen and bone marrow involvement.
A handful of cases with TB presenting along with HL have been reported in the literature till date. Some of them are being reported here [8-11]. Reddy RC et al., reported a case of 29-year-old woman, who presented with lymphadenopathy and was diagnosed and initiated on ATT based on the cervical node biopsy showing granulomatous lymphadenitis. Despite regular treatment, her condition worsened and an extensive work up revealed a diagnosis of Hodgkin’s disease of nodular sclerosis type [8]. Deeb S et al., reported a 15-year-old girl with significant weight loss and a rapidly growing chronic cervical mass, which showed simultaneously a classic HL on histopathology and TB-PCR positive [9].
Collu C et al., mentioned a case of 22-year-old Somalian boy with thoracic pain, weight loss, latero-cervical, mediastinal, and abdominal lymphadenopathy turned out to be a case of TB in culture, while other investigations were negative for TB suggesting lymphoma [10].
Göknar N et al., reported the case of a 14-year-old boy with fever, weight loss, and mediastinal lymphadenopathy. He was diagnosed with HL after several X-rays, computed tomography, positron emission tomography-computed tomography, laboratory tests and three lymph node biopsies [11]. All these cases suggest similar presentations of both the diseases as in our case, although differing in the age of presentation (childhood and adolescent populations) and the various investigational modalities used. The need of clinical suspicion and additional workup in case of non-improvement cannot be overemphasised.
Hyper eosinophilia seen in the patient is attributable to both HL and TB. Whereas, lymphomas are a well known cause of eosinophilia, the association of TB with it is rare yet reported in the literature. Garg G et al., reported a case of 68-year-old female who was diagnosed with TB and had peripheral eosinophilia which returned to normal levels after starting ATT [12]. Haftu H et al., described a case of nine-year-old female with hepatic TB with peripheral eosinophilia which resolved after three weeks of ATT [13]. In susceptible patients early hypersensitivity reactions to the mycobacterium antigen and raised IL-5 (an eosinophilic cytokine) levels have been proposed as mechanisms [14,15].
Conclusion(s)
This case brings to notice the need of identifying the two disorders in same patient as missing any one of them can be fatal. Whenever a patient diagnosed with TB doesn’t respond despite regular treatment, one has to think of drug resistance, atypical mycobacteria or malignancy. The importance of having a high index of suspicion cannot be overemphasised as both TB and HL are treatable and, in many cases, curable as well.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Dec 29, 2020
Manual Googling: Mar 15, 2021
iThenticate Software: Mar 22, 2021 (10%)
[1]. Jacobson CA, Longo DL, Hodgkin’s Lymphoma. In: Jameson JL, Kasper DL, Longo DL, Fauci AS, Hauser SL, Loscalzo J, editorsHarrison’s Principles of Internal Medicine 2018 20th edNew YorkMcGraw Hill:780 [Google Scholar]
[2]. Thakkar K, Ghaisas SM, Singh M, Lymphadenopathy: Differentiation between tuberculosis and other non-tuberculosis causes like follicular lymphomaFront Public Health 2016 4:3110.3389/fpubh.2016.0003126942176 [Google Scholar] [CrossRef] [PubMed]
[3]. Poppema S, Immunology of hodgkin’s diseaseBaillieres Clin Haematol 1996 9(3):447-57.10.1016/S0950-3536(96)80020-5 [Google Scholar] [CrossRef]
[4]. Kumar P, IFNγ-producing CD4+T lymphocytes: The double-edged swords in tuberculosisClin Transl Med 2017 6(1):2110.1186/s40169-017-0151-828646367 [Google Scholar] [CrossRef] [PubMed]
[5]. Sharma S, Sharma M, Roy S, Kumar P, Bose M, Mycobacterium tuberculosis induces high production of nitric oxide in coordination with production of tumour necrosis factor-alpha in patients with fresh active tuberculosis but not in MDR tuberculosisImmunol Cell Biol 2004 82(4):377-82.10.1111/j.0818-9641.2004.01245.x15283847 [Google Scholar] [CrossRef] [PubMed]
[6]. Shin DM, Yang CS, Lee JY, Lee SJ, Choi HH, Lee HM, Mycobacterium tuberculosis lipoprotein-induced association of TLR2 with protein kinase C zeta in lipid rafts contributes to reactive oxygen species-dependent inflammatory signalling in macrophagesCell Microbiol 2008 10(9):1893-905.10.1111/j.1462-5822.2008.01179.x18503635 [Google Scholar] [CrossRef] [PubMed]
[7]. Colović M, Colović R, Jovanović V, Petrović M, Perisić-Savić M, Tuberculosis of the lymph nodes and spleen preceding hodgkin’s diseaseSrpski arhiv za celokupno lekarstvo 1989 117(1-2):97-106. [Google Scholar]
[8]. Reddy RC, Mathew M, Parameswaran A, Narasimhan R, A case of concomitant hodgkin’s lymphoma with tuberculosisLung India 2014 31(1):59-62.10.4103/0970-2113.12598524669086 [Google Scholar] [CrossRef] [PubMed]
[9]. Deeb S, Ali T, Rehan H, Rare incidence of concomitant hodgkin’s lymphoma with tuberculous lymphadenitis: A case reportOtolaryngology Case Reports 2020 14:10014210.1016/j.xocr.2019.100142 [Google Scholar] [CrossRef]
[10]. Collu C, Fois A, Crivelli P, Tidore G, Fozza C, Sotgiu G, A case-report of a pulmonary tuberculosis with lymphadenopathy mimicking a lymphomaInt J Infect Dis 2018 70:38-41.10.1016/j.ijid.2018.02.01129477363 [Google Scholar] [CrossRef] [PubMed]
[11]. Göknar N, Çakır E, Çakır FB, Kasapcopur O, Yegen G, Gedik AH, A difficult case of hodgkin lymphoma with differential diagnosis of tuberculosis and sarcoidosisHematol Rep 2015 7(2):564410.4081/hr.2015.564426330996 [Google Scholar] [CrossRef] [PubMed]
[12]. Garg G, Gogia A, Kakar A, Miglani P, Persistent marked peripheral eosinophilia due to tuberculosis: A case reportIran J Med Sci 2017 42(1):102-05. [Google Scholar]
[13]. Haftu H, Tadese K, Gebrehiwot T, Gebregziabher H, How common is eosinophilia in tuberculosis? Case reportPediatric Health Med Ther 2020 11:59-63.10.2147/PHMT.S24415532110139 [Google Scholar] [CrossRef] [PubMed]
[14]. Ray D, Abel R, Hypereosinophilia in association with pulmonary tuberculosis in a rural population in south IndiaIndian J Med Res 1994 100:219-22. [Google Scholar]
[15]. Kolobovnikova IuV, Urazova OI, Novitskiĭ VV, Mikheeva KO, Goncharov MD, Molecular mechanisms of formation of blood eosinophilia under pulmonary tuberculosisVestn Ross Akad Med Nauk 2012 (5):58-62.10.15690/vramn.v67i5.276 [Google Scholar] [CrossRef]