Renal malignancies are common in children and they constitute 6-7% of all childhood tumours and nephroblastoma is the most common solid renal tumour in children [1,2]. Other malignant renal tumours are renal cell carcinomas, malignant rhabdoid tumours, clear cell sarcomas of the kidneys and the rarest of them all, renal lymphomas [2].
Nephroblastoma affects 1:10,000 children world-wide in those less than 15 years and an estimated 9/1,000,000 population of children between 0-9 years have nephroblastoma and it is also the most common abdominal tumour in sub-Saharan Africa [3-5]. About 4 cases are seen yearly in most tertiary hospitals in Africa however most of these cases present late [4]. Amongst all the primary renal tumours, nephroblastoma has the best prognosis especially the subtype with favourable histology. The success story in nephroblastoma particularly in high income countries has been made possible by the tremendous contributions of so many research groups, notably the Children Oncology Group (COG) in North America and the Societe international Oncologie Paediatrique (SIOP) in Europe. Over 85% of nephroblastomas are cured in high income countries but only 0-52.7% in African Countries [6,7]. The poor outcomes in low and middle income countries like Nigeria has been adduced to late presentations, non-completion of therapy, shortage of personnel and infrastructures and deaths related to treatments and diseases [4,5,7-9].
There are no standardised institutional protocols in management of renal tumours in children in many African countries [7]. Even though some large scale collaborative studies have started emerging, not much has been documented on the pathology, clinical presentation and outcome of solid renal tumours and nephroblastoma in particular in Africa.
A paediatric oncologic group was recently formed and there was a need to audit previous practice as a preliminary. Therefore, the objective of the study was to add the existing body of knowledge of solid renal tumours and nephroblastoma in particular from a low and middle income country perspective. It was also to determine the demographic profile of children with solid renal tumours in our setting, its clinical and pathological characteristics and to evaluate the treatment outcomes and factors affecting it. This shall help for future treatment modifications with the aim of improving outcomes. Above all this study intends to emphasise the place of an adopted institutional protocol and multidisciplinary collaboration in the management of this condition and for good outcome.
Materials and Methods
This was a nine and a half year (January 2009-June 2018) longitudinal retrospective audit study of consecutive children who were diagnosed of and managed for solid renal tumours at Nnamdi Azikiwe University Teaching Hospital (NAUTH) Nnewi, a tertiary health centre, South East Nigeria. A waiver was obtained from the Institutional Ethics Committee for this study (NAUTHEC/2021/083).
Inclusion criteria: Paediatric renal tumour data or records confirmed by radiography or ultrasonography or histology, who were completely or incompletely managed in the hospital were included in the study.
Exclusion criteria: The data of patients above 16 years of age and patients who were not managed at all by any of the collaborating units were excluded from the study.
A list of paediatric patients with solid renal tumours were compiled from the operating theatre registers and the patients records in the paediatric surgery and paediatric medical wards. The folders of the cases in the list were retrieved from the Medical Records Department of the hospital. Histopathology results were retrieved from the folders and were not available, from the Histopathology Department of the hospital. From the folders, relevant data on demography, clinical features, histopathologic characteristics, investigations, treatments and outcome were extracted. This audit report complied with the Helsinki declaration on human studies of 1975 as revised in 2013.
Statistical Analysis
The data were then keyed into SPSS spreadsheet. Results were analysed using statistical software package for social sciences (SPSS version 22) and presented in means, percentages and tables. Categorical data were tested for association using chi-square test and significance inferred at a p-value of <0.05.
Results
Twenty two cases met the inclusion criteria within the study period and their records were extracted and evaluated. The mean age at presentation was 50.10±45.18 months [Table/Fig-1]. There were 15 males and 7 females, giving a M:F ratio of 2.1:1. The mean duration of symptoms was 5.5 months [Table/Fig-1] and 21 (95.5%) presented with abdominal masses while 6 (27.3%) had gross haematuria and 13 (59.1%) were emaciated [Table/Fig-2]. Herbal medications and scarifications had been administered to 15 (68.2%) and 6 (27.3%), respectively. There was an association between delayed presentation and abdominal mass, (χ2=6.635, p=0.01); however with Fisher-exact test, it was not significant, p=0.136 [Table/Fig-3]. There was no association between delayed presentation and gross haematuria, cough, herbal medication and weight loss; (χ2=1.303, χ2=0.222, χ2=1.945, χ2=0.953; p>0.05; respectively). There was also no association between gender and abdominal pain [Table/Fig-3]. There were no associated congenital anomalies like aniridia, undescended testis, and ambiguous genitalia. Also, there were no associated acquired groin anomalies like varicocele, and hernia.
Patients’ characteristics.
| Variables | Min | Max | Mean |
|---|
| Age at presentation (months); n=22 | 8.0 | 168.0 | 50.09 (±45.181) |
| Age at onset of symptoms (months); n=22 | 5.0 | 168.0 | 46.05 (±46.474) |
| Duration of symptoms (weeks); n=22 | 0.40 | 152.00 | 22.34 (±35.972) |
| Systolic blood pressure; (mm of Hg) n=15 | 80 | 150 | 110.9 (±18.79) |
| Diastolic blood pressure; (mm of Hg) n=14 | 50 | 90 | 68.6 (±14.06) |
| Weight in Kg; n=19 | 2 | 30 | 14.3 (±6.36) |
| Height in cm; n=13 | 45 | 135 | 90.2 (±24.76) |
Common clinical features of patient.
| Symptoms | Yes (%) | No (%) | Total |
|---|
| Abdominal mass | 21 (95.5) | 1 (4.5) | 22 |
| Abdominal pain | 12 (54.5) | 10 (45.5) | 22 |
| Weight loss | 13 (59.1) | 9 (40.9) | 22 |
| Fever | 8 (36.4) | 14 (63.6) | 22 |
| Gross hematuria | 6 (27.3) | 16 (72.7) | 22 |
| Microscopic haematuria | 5 (22.7) | 7 (77.3) | 12 |
| Cough | 5 (22.7) | 17 (77.3) | 22 |
| Herbal medication | 15 (68.2) | 7 (31.8) | 22 |
| Pallor | 8 (36.4%) | 12 (53.6%) | 20 |
Association of delayed presentation with some patient’s parameters.
| Correlated parameters | Pearson Chi-square value | p-value |
|---|
| Abdominal mass | 6.635 | 0.01 |
| 0.136* |
| Gross haematuria | 1.303 | 0.254 |
| Cough | 0.222 | 0.637 |
| Herbal medication | 1.945 | 0.163 |
| Weight loss | 0.953 | 0.329 |
| Gender and abdominal pain** | 1.180 | 0.277** |
*Fisher-exact test **Association between gender and abdominal pain
Tumours were on the left kidney in 15 (68.2%) cases and on the right side in 7 (31.8%) cases. Tumours have crossed the midline in 7 cases (31.8%), while 11 cases did not cross the midline. In 7 patients (31.8%), tumour have smooth surface, while 6 patients (27.3%) have nodular surfaces. The rest 9 had no comment on the status of their tumour surface. Six (27.3%) patients have received abdominal scarifications from traditional doctors before presentation. The mean haemoglobin level was 8.99 (±2.039) g/dL, implying that most patients were anaemic. The creatinine levels were normal, with a mean of 62.7 (±21.99) umol/L.
Ultrasound was the most common imaging modality used in the patients and it was quite helpful in detecting the presence of a kidney mass and the laterality of the mass. However, the reports were lacking some vital information. In the reports, only 4 mentioned the lobe of the kidney involved, 12 made no comment on capsular invasion, 13 had no report on intracaval extension, 20 did not report about lymph node involvement, 4 were silent on the contralateral kidney, 4 also did make comment on liver involvement, and likewise 5 about ascites.
Of the 22 patients seen with solid renal neoplasm, 15 had an operative procedure. Nephroureterectomy was carried out on 11 (50%) while 2 were unresectable and one each was discharged against medical advice and biopsy respectively [Table/Fig-4]. The stages of the tumours were: stage I (1; 4.5%); stage II (3; 13.6%); stage III (10; 45.5%); stage IV (0; 0%); and stage V (2; 9.1%). Other intraoperative findings are shown in [Table/Fig-5]. There was inadequate under-reportage of essential intraoperative findings necessary for adjuvant therapy and prognostication.
Patients and surgery done.
| Surgery done | Frequency | Percentage (%) |
|---|
| Nephroureterectomy | 11 | 50 |
| Open biopsy then nephroureterectomy | 1 | 4.5 |
| Open biopsy for left and nephroureterectomy for right | 1 | 4.5 |
| Non-resectable | 2 | 9.1 |
| Not operated on | 6 | 27.3 |
| DAMA | 1 | 4.5 |
DAMA: Discharge after medical advice
Other intraoperative findings.
| Findings | Yes | No | No report |
|---|
| Lymph node status | 5 involved | 2 free | 8 |
| Intra-op spill | 5 | 9 | 1 |
| Extension into renal vein | 3 | 10 | 2 |
| Extension into IVC | 3 | 10 | 2 |
| Capsular breech | 3 | 7 | 5 |
| Lymph node type | Hilar-1; para-aortic-5 | |
| Liver | 0 | 13 | 2 |
| Contralateral kidney | 1 | 13 | 1 |
Histological report was available in 9 patients with some degrees of incomplete reporting. The histologic types reported were as follows: nephroblastoma (4); favourable Wilms tumour (2); rhabdomyomatous wilms (1); clear cell sarcoma (1); and renal cell carcinoma (1). Grading, presence or absence of anaplasia, tumour free margin were reported in one case each [Table/Fig-5,6]. Lymph node status was reported by the histopathologist in 3 cases.
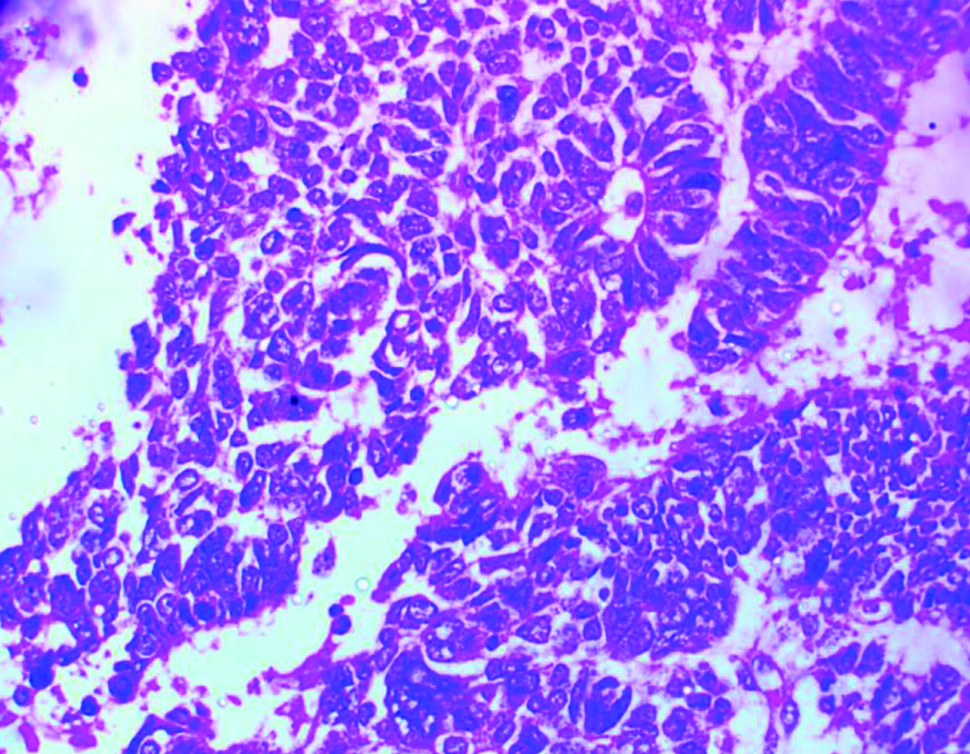
Photomicrograph of a nephroblastoma showing sheets of blastema cells with some anaplasia (H&E x400).
The protocol of management of the patient was Children Oncology Group (COG) in 6 and International Society of Paediatric Oncology (SIOP) in 6, while the remaining 10 did not follow a clear cut protocol [Table/Fig-7] [4,6]. The chemotherapy regimen and durations (both neo-adjuvant and adjuvant) were also varied. These are shown on [Table/Fig-7].
| Parameters | Neo-adjuvant therapy | Adjuvant therapy |
|---|
| Number of patients | 7 | 14 |
| No. of courses | 1-4; 3.5 (±2.35) | 1-13; 6.8 (±3.82) |
| Duration (weeks) | 4-14; 8 (±4.2) | 4-76; 30 (±24.3) |
| Drugs used | VAD–5, VACD–1 | VAC-1, VACD-3, VAD-5, VA-1, VCDE-1 |
| Protocol used | n |
| COG | 6 |
| SIOP | 6 |
| No defined protocol | 10 |
COG: Children oncology group; SIOP: International society of paediatric oncology; V: Vincristine; A: Adriamycin; D: Dexamethasone; C: Cyclophosphamide; E: Etoposide
There were 5 mortalities during treatment. The causes of death were advanced nephroblastoma in 2, recurrence in 1 and tumour emboli in 2. The average age at death was 55.4 (±45.1) months. Six patients were alive to completion of treatment. Duration of follow-up was 8 to 48 months (22.5±16.41) for patients who completed treatment. Other patients were not completely accounted for: four defaulted during follow-up, and five were completely lost to follow-up. Four of the treated patients had recurrence.
Discussion
Amongst all the primary renal tumours, nephroblastoma has the best prognosis especially the subtype with favourable histology. Over 85% of nephroblastomas are cured in high income countries but only 0-52.7% in African Countries. The success story in nephroblastoma particularly in high income countries has been made possible by the tremendous contributions of so many research groups, notably the COG in North America and the SIOP in Europe [6,7]. The poor outcome in Lower and Middle Income Country (LMIC) had been attributed to delayed presentation, shortage of personnel and infrastructures, non-completion of therapy, and deaths related to treatments and diseases [4,5,7-9]. However, adequate attention has not been given to the effect of implementation or not of a strict institutional protocol (which are results research groups works) and multidisciplinary collaboration in the management-outcome of these conditions [7].
There were a total of 22 children with malignant solid renal tumours however, only 11 (50%) had complete records, a finding similar to the experience of Atanda AT et al., who had 18 (60%) of their 30 patients with complete records. This finding is quite common in developing countries of the sub-Saharan Africa and record keeping needs to be improved [5].
The age range of the patients was 8 months-14 years with a mean of 50.1 months ±45.18 similar to findings by Atanda AT et al., and Woldeab WE et al., [5,10] but older than that in the National Wilms Tumour Study Group (NWTSG) of median age of 38 months [3]. The male to female ratio in this present study is 2.1:1 and it is similar to findings in previous reports from Nigeria [5,7].
Nephroblastoma is the most common malignant tumour of the kidney [2,11]. Others are much rarer and include renal cell carcinoma, malignant rhabdoid tumour, clear cell sarcoma and renal lymphomas [1,2]. In the previous study on childhood cancers in the sub-region, nephroblastoma ranked highest as the most common malignant tumours alongside lymphomas, leukaemias, rhabdomyosarcomas with a tied percentage frequencies of 11.6% of all childhood malignancies [12]. The mean duration of symptoms amongst our patients was 5.5 months and this is late though shorter than the findings of 9 months reported by Atanda AT et al., and Anyanwu LJ et al., both in northern Nigeria [5,7]. Wilde JC et al., in a Malawian study reported a mean duration of symptoms of 3.2 months and Ekenze SO et al., in South Eastern Nigeria [13,14]. These variations may be because of difference in the health seeking behaviours of patients across the different regions. This late presentation remains a challenge in managing malignant renal tumours in children in Africa [1,14].
The most common presenting complaint was abdominal masses in 21 (95.5%) and this is same with previous studies [5,10,15]. This emphasises the need for detailed evaluation of all children with abdominal masses in centres by experienced paediatricians. Other common features in these patients were haematuria in 6 (27.3%), fever in 8 (36.4%) and weight loss in 13 (59.1%). The large number of those that were already emaciated, further suggests late presentation in our setting and negatively affects outcome. Part of the delays in presentation is as a result of delayed referrals from the referring centres or as a result of unorthodox cares. Authors found that 6 (27.3%) and 15 (68.22%) had various forms of abdominal scarifications and herbal concoctions respectively. These unorthodox practices have been highlighted by Osuoji RI et al., in Benin city Nigeria as a major cause of delay in presentation of children with abdominal masses [15]. Sequel to these late presentations, 8 (36.4%) were anaemic and required blood transfusions before treatments.
Authors had more of the tumours in the left kidney and this is similar to previous studies [5,7]. The reason for more of left prevalence is not yet known. Authors used abdomino-pelvic ultrasound scan and intravenous urography for evaluations and these were helpful in determining the presence of renal tumours but did not help in determining operability. We believe that further imaging with computerised tomographic scan and/or magnetic resonance imaging is needed to guide tumour staging and therapeutic decisions [3]. These were largely unavailable or unaffordable in most of the durations covered by this study.
As a result of late presentations, majority of the patients 10 (45.5%) presented with late stages of the disease while both stage I and II were seen in (18.8%). This finding is not uncommon in similar studies from Africa [4,5,7,9,10,16]. The staging was done after surgery with combination of intraoperative findings and clinical features. Nephroureterctomy without neoadjuvant chemotherapy was carried out on 11 (50%) of the patients who were later commenced on adjuvant chemotherapy. Two of the tumours were not resectable and the patient with bilateral tumour was discharged following an industrial action and the patient did not present to our facility thereafter. A patient had an open biopsy and nephroureterctomy after a neoadjuvant chemotherapy.
The attitude of late presentation also reflected in compliance with treatment and follow-up [14]. Several of the patients dropped out in the course of treatment and follow-up. It is noted that of the 22 presenting patients, only six could be accounted for as either being alive or dead, 1 to 4 years after treatment. It seems most people in the study population are still steeped in ignorance and superstitious believes of cause of certain ailments. Moreover, the high cost of healthcare and out-of-pocket payment coupled rank high amongst other factors that contributes to late presentation of patients to orthodox health care facilities. These were similar to reports in other similar centres [7,14].
The absence of protocol and collaboration was quite glaring in the care of these patients. Management of any malignancy should be multi disciplinary. The report of ultrasound from the radiologist shows that there is discordance between the desired information needed from them and the information they document in their report for cases of solid renal neoplasm. For example, report of whether there is or not renal vein and intra-caval extension of neoplasm is key in determining whether to give neo-adjuvant chemotherapy in COG protocol. It also aids surgical preparation vis-a-vis getting a vascular surgeon involved. This may have accounted for the mortalities arising from intraoperative tumour emboli. Also, lobes/poles of the kidney involved when reported could give an idea of the possibility of nephron-sparing surgery especially in bilateral tumours or in the case of a single functioning kidney. However, one expected that a better imaging modality, such as contrast enhanced CT-scan will be used in such cases. A regular discussion with the radiologists and incorporating them as part of the oncology team will help to close this gap. Anyanwu LJ et al., emphasised the importance of this collaboration in his study even to the point of involving pharmaceutical company [7].
It was also noted that there exist an inconsistency in the protocol used in patient management. Some cases had SIOP, others had COG, and the rest had an undefined protocol of care. This arises from cases of renal tumour presenting to and being handled by either paediatric oncology or paediatric surgery team. This shows there is no protocol and solid collaboration in patient management. The era of single-discipline care is no longer fashionable. This is because it does not allow for optimal care, outcome measure and proper comparison of outcome. Rabeh W et al., and Ekenze SO et al., had emphasised how multi-disciplinary collaboration involving every person connected to patients management leads improvement in overall outcome in a LMIC [17,18].
Moreover, inter-surgeon approach to operation was also discordant. Intraoperative findings were highly inadequate, and this makes for questionable staging. It should be understood that a proper surgery also includes a proper documentation. It makes research tenable. The omission of lymph node status was quite alarming. The adoption of a protocol and collaboration will minimise this. The involvement of many disciplines who will be interested in what each has done, and how it affects other practices will ensure that some omissions are guarded against [17]. If a radiologist needs to know the predictive nature of his ultrasonography in detecting lymph node involvement, and a pathologist needs to analyse a harvested lymph node, then a surgeon will not fail to harvest one and to document so.
It will also be noted that the histopathology reports was also inadequate. It again points to absence of collaboration. The management of childhood solid renal neoplasm has evolved. There have been changes in terminologies and histology. Each nephroblastoma should be sub-typed. And reference must be made as to the presence or absence of anaplasia. These affects adjuvant treatment and further care, and for prognostication. Frequent discussion and collaboration would bring everybody to speed and to expectation.
Limitation(s)
Besides small sample size, this study was also limited by incomplete and lost patients’ data and records. Discordant protocol and uncoordinated management ensured that there was no consistency in data and made comparison difficult. It is also limited by its retrospective nature.
Conclusion(s)
The pattern of presentations and outcome in this study has not varied from similar review in other similar centres. The management of solid childhood neoplasm in our centre, like in other developing countries, is riddled with a lot of challenges. Outcome has not been good. However we believe that if there is an adoption of a standard binding protocol and multi-disciplinary collaboration are instituted, outcome will improve. Then going forward, other challenges could be addressed.
*Fisher-exact test **Association between gender and abdominal pain
DAMA: Discharge after medical advice
COG: Children oncology group; SIOP: International society of paediatric oncology; V: Vincristine; A: Adriamycin; D: Dexamethasone; C: Cyclophosphamide; E: Etoposide