Globally, the second most common type of cancer is Prostate Carcinoma (PCa) and is observed as sixth cause of cancer deaths among men [1]. According to statistics, there were about 1.3 million patients of prostate cancer in 2018 and the global PCa load is expected to increase to 1.7 million new cases and 499,000 casualties by 2030. This is the result of growth and increased life expectancy of the population along with environmental factors [2,3].
Sedentary lifestyle, genetics and age are long established determinants of PCa. In carcinogenesis chronic inflammation plays a pivotal part. In PCa aetiopathogenesis, besides genetics and environmental factors; infection, diet, and other exposures like radiation contributes to chronic prostate inflammation [4]. Risk factors associated with modernised lifestyle and economic expansion which play a role in increasing this incidence are higher consumption of animal fat, obesity and physical inactivity [5].
Inspite of the growing corroboration, PCa related mortality rate is decreasing. The improved biopsy techniques and Prostate Specific Antigen (PSA) estimation are two major tools for its explaination. [6]. The gold standard method in detection of PCa is TRUS guided prostate biopsy. PSA is a member of kallikrein-related peptidase family which has been universally used for early detection of PCa. Elevated levels of PSA are the most regular genesis for prostate biopsy. However, PSA screening also results in unnecessary biopsies, especially among patients in grey zone (PSA more than 4 ng/mL but less than 10 ng/mL). On the other hand, in introductory prostate biopsy, approximately one out of five men with PCa might be misdiagnosed [7].
Therefore, there is clear need of novel markers, which can detect both clinically significant PCa and prevent unnecessary biopsies. Among these markers, there may be connection of higher NLR with neutrophil dependent body’s response to inflammation and lower lymphocyte levels with lowered antitumoural response which may correspond to faulty prognosis related to aggressive tumour biology and progression of cancer [8]. Also, some studies suggest that increased neutrophil decrease anti-tumoural immune reaction [9]. Inflammatory response of body has been revealed as a self-sufficient medium to predict oncological outcomes [10].
Still some researchers have signalled that tumour load is connected to thrombocytosis [11]. Platelets release PDGF and thrombospondin. PDGF is a potent mitogen while thrombospondin, when acts like an adhesive glucoprotein; enhance the adhesive nature of tumour cells [12]. Though these facts seem to explain the cause effect relationship between platelets and carcinogenesis, insufficiency of satisfactory information still exists.
Variation in volume and size of red blood cells is measured by RDW. Literature shows that RDW has strong relationship with factors of inflammation such as erythrocyte sedimentation rate, C-reactive protein and fibrinogen [13].
Therefore, the aim of this study was to find a parameter which will be less expensive and easily available, helpful in making clinical diagnosis, follow-up and predicting prognosis in prostate cancer and benign pathology. The first objective was to study the significance of pretreatment NLR, RDW, PSA and PLR in identifying PCa group and group with benign enlargement of prostate. Second objective was to observe the impact of pre-treatment NLR, PLR, PSA and RDW on PCa prognosis.
Materials and Methods
This cross-sectional study was conducted in the Department of Pathology, Geetanjali Medical College and Hospital, Udaipur, Rajasthan between January 2018 to November 2020 and included 84 patients who underwent multicore TRUS-guided prostate biopsy. The present study was in accordance with the Helsinki Declaration and it did not require ethics committee permission as it included retrospective data. Among all males, the prostate was routinely biopsied by transrectal route under local anaesthesia following preoperated administration of a single dose antibiotic prophylaxis and gastrointestinal cleaning.
Inclusion criteria: All the biopsy samples received in the pathology department were included during study period.
Exclusion criteria: Patients with any history of an autoimmune or inflammatory disease, myeloproliferative disorders, splenectomies, urinary tract infections or anti-inflammatory drug use were excluded.
Biopsy specimens were evaluated in the histopathology section of pathology department. In patients with established diagnosis of PCa: PSA levels, neutrophil and platelet counts, prebiopsy whole blood cell counts, Gleason scores (Gs) and biopsy results were evaluated [9]. Division of absolute neutrophil count by absolute lymphocyte count for NLR and platelet count by lymphocyte count for PLR. RDW was taken from CBC report. The three groups among non-PCa i.e., Benign Prostatic Hyperplasia (BPH), prostatitis and PCa were categorised by histological results.
Statistical Analysis
Frequencies and percentage were calculated for the categorical data and measures of central tendency i.e., Mean or Median and standard deviation was used for continuous variables. Between two groups, the comparison of variables was performed according to the normality either unpaired t-test or Mann-Whitney U test for continuous variable. To evaluate the possible association between the PSA, NLR, PLR, RDW and Gleason score Logistic regression was used. The p-value less than 0.05 considered as statistically significant. Sensitivity and specificity of the study parameters in PCa detection was assessed using ROC analysis. The data was statistically analysed using IBM SPSS version 20.0.
Results
Eighty four patients who underwent TRUS guided prostate biopsy were considered. These cases were firstly categorised as non-PCa and PCa. Out of 84 patients, 50 were in the benign category (non-PCa) and 34 were PCa patients. Out of those 50 non-PCa patients, 10 were chronic prostatitis and 40 were having BPH. Age, PSA levels, NLR, PLR and RDW of all the patients with different categories are presented in [Table/Fig-1].
| Variables (Mean±SD) | PSA value |
|---|
| Non-PCa (n=50) | PCa (n=34) |
|---|
| Chronic Prostatitis (n=10) | BPH (n=40) |
|---|
| 4-10 | >10 | 0-4 | 4-10 | >10 | 4-10 | >10 |
|---|
| Age (in years) | 68±7.87 | 68.75±7.08 | 65.35±7.92 | 73±9.06 | 67±2.82 | 64.8±7.25 | 69.32±7.77 |
| PSA | 8.45±1.73 | 19.77±6.27 | 1.02±1.10 | 5.41±1.22 | 18.67±11.78 | 7.16±1.77 | 63.86±34.58 |
| NLR | 3.93±1.29 | 5.46±1.44 | 2.93±1.34 | 4.10±3.49 | 4.59±1.46 | 2.76±1.09 | 3.04±1.76 |
| PLR | 181.63±50.20 | 220.66±112.35 | 145.46±69.12 | 154.12±55.67 | 148.07±28.79 | 140.54±50.26 | 134.36±64.40 |
| RDW | 15.24±2.02 | 16.87±0.90 | 14.16±2.76 | 15.46±2.37 | 15.5±0.56 | 15.5±3.99 | 14.78±3.52 |
The median NLR in non-PCa group was significantly higher than PCa group (p=0.149). Median PLR and RDW values in non-PCa and PCa groups are represented in [Table/Fig-2], respectively and there was statistically insignificant difference in PLR (p=0.070) and RDW (p=0.4413) values among both the groups.
Test of significant difference of NLR, PLR, RDW in non-PCa and PCa cases.
| Parameter | Non-PCa n=50 | PCA n=34 | p-value |
|---|
| Age (Mean±SD) | 67.44±8.49 | 68.7±7.67 | 0.4898* |
| PSA | 2.55 (0-25) | 52.09 (0.57-100) | <0.001** |
| NLR | 3.31 (0.846-10.851) | 2.496 (0.822-8.028) | 0.149** |
| PLR | 153.12 (27.75-373.41) | 125.57 (42.64-297.24) | 0.070** |
| RDW | 14.85 (10.5-21.5) | 14 (11-25) | 0.4413** |
*Unpaired t-test; **Mann-Whitney U test, p<0.05 considered as level of significance
[Table/Fig-2] shows the association between PSA, NLR, PLR, RDW values and PCa detection. Serum PSA score was found to be significantly higher in the PCa group as compared to non-PCa group (as BPH and prostatitis are both benign conditions so are kept in non-PCa group) (p<0.001). There was no statistically significant difference in NLR (p=0.149), PLR (p=0.070) and RDW (p=0.4413) values among non-PCA and PCa group.
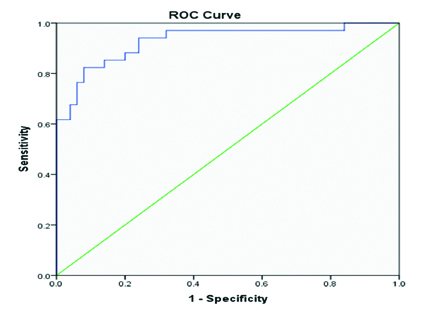
Out of total 34 patients of PCa group maximum nine patients (26.47%) were in grade 2 (GS-3+4=7) followed by eight patients (23.53%) in Grade 5 (GS ≥9). Distribution of PCa patients according to gleason score grading is given in [Table/Fig-3]. Sensitivity and specificity of PSA, NLR, PLR and RDW are presented in [Table/Fig-4], respectively. Sensitivity and specificity of the study parameters in PCa detection was assessed using ROC analysis [Table/Fig-5,6].
Distribution of PCa patients according to gleason score grading.
| Gleason score (GS) | Grade | Number of patients (%) |
|---|
| 3+3=6 | 1 | 5 (14.71) |
| 3+4=7 | 2 | 9 (26.47) |
| 4+3=7 | 3 | 6 (17.65) |
| 4+4=8, 3+5=8 and 5+3=8 | 4 | 6 (17.65) |
| 5+4=9, 4+5=9 and 5+5=10 | 5 | 8 (23.53) |
| Total | 34 (100) |
Cut-off values, Sensitivity and Specificity of different parameters.
| Parameters | Cut-off | Sensitivity | Specificity |
|---|
| NLR | 3.04 | 61.76% | 58% |
| PLR | 97.40 | 38.24% | 86% |
| RDW | 13.65 | 47.06% | 68% |
| PSA | 12.63 | 82.35% | 92% |
ROC curve for NLR, PLR, RDW.
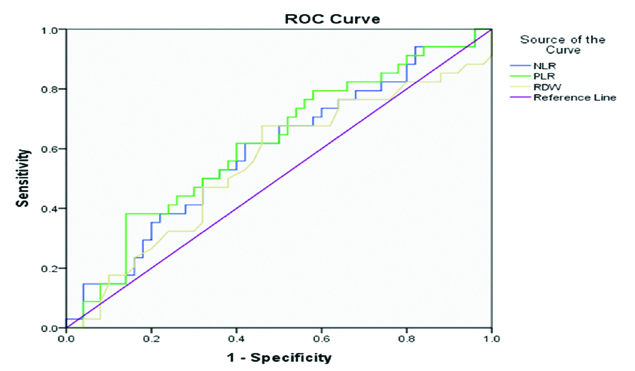
Statistically insignificant correlation was observed between Gleason Score, NLR, PLR and RDW in PCa group. Correlation observed between PSA and Gleason Score was statistically insignificant [Table/Fig-7].
Correlation analysis of NLR, PLR and Gleason Scores with PSA value of PCa and non-PCa (BPH and Chronic prostatitis).
| Parameters | PSA score {r (p-value)} | Gleason Score {r (p-value)} |
|---|
| BPH | Prostatic carcinonoma | Chronic Prostatitis |
|---|
| NLR | 0.11 (0.05) | 0.0004 (0.99) | 0.76 (0.01) | -0.19 (0.26) |
| PLR | 0.09 (0.57) | -0.18 (0.31) | 0.09 (0.80) | -0.25 (0.14) |
| RDW | 0.24 (0.14) | -0.11 (0.55) | 0.52 (0.13) | -0.14 (0.43) |
| PSA and gleason score | 0.12 (0.51) |
r is correlation coefficient
Discussion
The standard examination on patients suspected with PCa is Serum PSA level and prostate biopsy. PSA measurement is most commonly used as screening marker for PCa and its level is observed to be increased in blood due to destruction of the integrity of prostatic glands. Hence, PSA is released into the blood in benign conditions like chronic prostatitis other than PCa [14,15]. In a survey, performed by National Health and Nutrition Examination Survey Prostate (NHANES) on 3164 healthy men of age >40 years, with absence of prostatic disease, a significant relation was established between systemic inflammation markers and increased serum PSA levels of ≥4 ng/mL [16]. Although, PSA levels=4 ng/mL are widely accepted as a threshold level, since PCa has also been diagnosed in patients with serum PSA levels <4 ng/mL. PSA marker is specific for prostate organ but not for cancer. In this study, a significant association of serum PSA level and PCa with higher Gleason score is obviously seen, but, in addition, raised levels are also seen in prostatitis.
Only 20-67% prostatic cancers are diagnosed by biopsy [17]. Thus, to detect cancer, recurrent biopsies are a prerequisite [18,19]. To prevent unrequired biopsies and biopsy related complications, cheap and widely used markers are needed. Therefore, we have used less expensive and easily available parameters like NLR, RDW and PLR, which are routinely checked during CBC examination, and made them part of this study.
In many different solid organ tumour, the predictive value of NLR has been observed in both prognosis and carcinogenesis [20]. Ergin G et al., and Langsenlehner T et al., observed significant association between high levels of NLR and Gleason scores in their studies [21,22]. In addition to this, Langsenlehner T et al., also concluded that high NLR is significantly associated with prostate carcinogenesis [22]. Study performed by Anggara R et al., showed an important relationship between CBC parameter and Gleason score in prostate cancer patients [23]. Likewise Huang TB et al., also indicated strong relationship between NLR and PCa detection when PSA ranged from 4 to 10 ng/mL [7]. Beneficial role of CBC parameters in predicting progression and prognosis of PCa was also observed by Sun Z et al., in his study performed on 226 PCa patients [24]. The results of this study are not similiar to those of Ergin G et al., and Langsenlehner T et at., and observed negative correlation between NLR and Gleason’s score [21,22].
Zanaty MAK et al., in his study, found insignificant association of NLR and PLR levels among organ confined patients with prostate cancer, and concluded that localised tumours may not be the result of systemic inflammatory response [25]. Likewise, in this study, we were unable to find a significant association between NLR and PCa.
Another study conducted in Japan revealed that increased neutrophil count was significantly associated with increased chances of a benign prostate biopsy [26]. Yuksel OH et al., in his study reviewed total of 873 patients who undergone prostate biopsy and observed that NLR value in PCa patients was similar with non-PCa patients (p=0.944) [9]. In the present study, NLR, an inflammatory marker, revealed no difference regarding non-PCa and PCa (p=0.149). This may be due to known reasons like: in approximately 20% of cases of BPH, inflammation and prostate cancer co-exist in the one prostate zone [27]. Gokce MI et al., in his study compared the distribution of NLR among PCa patients, prostatitis and BPH, and observed that prostatitis presence may limit the use of NLR in predicting PCa [28]. Moreover, there are some contradictory evidences in literature reports regarding prediction value of PLR for diagnosis and PCa prognosis [29].
PLR valued as an important prediction marker for differentiation between benign prostate lesions and malignant ones in study done by Yuksel OH et al., and Wang Y et al., [9,30]. On the other hand, study performed by Zanaty MAK et al., were not able to observe noteworthy connection between PLR value and PCa [25]. In the present study also, we could not find a significant association between PCa and PLR or an increment of Gleason score to an increment in the PLR.
Meta-analysis performed by Patel KV et al., concluded that RDW as an effective indicator with cancer associated deaths [31]. In a study of Seretis C et al., significant association of higher RDW in invasive breast cancer patients than patients with fibroadenomas was reported [29].
Albayrak S et al., concluded higher RDW in PCa patients than those in healthy controls [32]. In present study, we could not find any important relationship between RDW and PSA, RDW and Gleason score and it was statistically insignificant.
Limitation(s)
This study was limited by the possibility of referral bias and population migration. The size was low, thus choice for statistical test was limited. As discussed before, 20% of PCa may be misdiagnosed in initial biopsy, thus patient having malignancy (PCa) may be diagnosed as benign.
Conclusion(s)
Various studies have been performed trying to relate PCa to diverse markers so as to provide its easy and early detection. Through analysis, it was concluded that these studies reveal conflicting results, and even though PSA is an important marker in prostate cancer diagnosis, markers like NLR, PLR and RDW cannot be fully trusted to be accurate in prognosis of PCa and differentiating it from other benign conditions like BPH and chronic prostatitis. Hence, there is still need for finding diverse markers to give relevant and high probability results in correct discernment of PCa.
*Unpaired t-test; **Mann-Whitney U test, p<0.05 considered as level of significance
r is correlation coefficient