Introduction
Cancer of uterine cervix comprises a big chunk of cancer registration worldwide. Now-a-days the immunohistochemical marker p16 has emerged as the surrogate marker of high risk Human Papilloma Virus (HPV) infection in cervical tissue. Galectin-3, a ubiquitous agent likely to modulate different pro-survival properties necessary for neoplastic cells, is recently emerging as the guardian of tumour microenvironment.
Aim
To study the expression of p16 and galectin-3 in different histomorphological variants of cervical Squamous Cell Carcinoma (SCC) and their association with grade and stage.
Materials and Methods
An observational cross-sectional study was undertaken in the Department of Pathology in a tertiary care hospital in East India, from January 2019 to June 2020. Fifty three samples diagnosed as invasive Squamous Cell Carcinoma (SCC) of uterine cervix were taken by systematic random sampling. Immunohistochemical examination was done using monoclonal antibodies against p16 and galectin-3 after obtaining thin sections from formalin fixed paraffin embedded blocks and retrieval of antigen. The data was interpreted by light microscopy using a semi-quantitative method with respect to prefixed parameters and statistical analysis was done by Chi-square test and Fisher’s exact test using Statistical Package for the Social Science (SPSS) version 25.0.
Results
Fifty two out of fifty three cases (98.1%) of squamous cell carcinoma in this study showed almost 100% block posivity of p16 in the tumour cells -strongly corroborative with high risk HPV infection. The non-keratinizing and the basaloid variant showed the strongest intensity of staining (3+). Only one case showed complete negativity of p16 expression. In galectin-3 positive cases, strong expression of this marker is found in the invasive tongues of the tumour cells at the junction of tumour stromal interface, consistent with our knowledge regarding the importance of galectin-3 in regulating the tumour microenvironment. The strongest galectin-3 positivity(3+) was found in the single case of Lymphoepithelioma like squamous cell carcinoma and showed almost 100% positivity among the neoplastic cell population; whereas the non-keratinizing and Basaloid variant showed almost negative expression. Significant association (p=0.00021) found between tumour grade and p16 intensity.
Conclusion
The non-keratinizing and basaloid variants of squamous cell carcinoma have shown statistically significant association with highest intensity of p16 staining along with diminished expression of galectin-3. Increased tumour grade is also significantly associated with strong staining intensity of p16 and decreased galectin-3 expression. However, no significant association is found between galectin-3 expression or intensity of p16 expression and the stage of tumour.
Introduction
Cancer of uterine cervix is the second or third most common cancer in women with approximately 0.5 million cases worldwide [1]. Total 76% of recent cases occur in low resource nations [1] and in developing countries like India.
The recognition of cervical premalignant conditions, their association with high risk human papilloma virus subtypes have made amenable the disease for screening and prevention. The introduction of Papanicolaou (PAP) smear screening in the women of reproductive age group as well as routine vaccination against high risk HPVs in adolecscent girls have infact, revolutionised the approach of managing this killer disease in western countries. However, lack of these public health measures in our country to the population who deserve it most; leads to the diagnosis of Carcinoma (ca) cervix in florid invasive stage most often; and so even in this era, it appears to be a major community health problem in the non-communicable chronic disease category in this part of the world [1].
Among the different morphological variants, squamous cell carcinoma is the most common type of cervical cancer [1] and is well known to evolve through definite premalignant conditions known as cervical intra-epithelial neoplasms or squamous in-situ lesions. There are eight recognized histomorphological variants of cervical SCC namely 1. Keratinizing 2. Non-Keratinizing 3. Basaloid 4. Warty 5. Papillary 6. Verrucous 7. Squamotransitional 8. Lymphoepithelioma Like [1]. The role of high risk HPV infections (HPV 16 and 18) among atleast 70 genetically distinct type of HPVs [2], the pathogenesis of SCC cervix is now well established [3].
So far the the pathogenesis of ca cervix is known to progress after integration of high risk HPV genome to the host genome, leading to interruption of viral Deoxyribonucleic Acid (DNA) within E1/E2 reading frame, loss of E2 viral repressor and increased expression of E6 and E7 oncoproteins [4]. The E6 protein binds and mediates the degradation of p53 [5] and stimulates the expression of Telomerase Reverse Transcriptase (TERT). E7 binds to Retinoblastoma (RB) protein in the active hypophosphorylated form which normally sequesters transcription factor E2F in the same binding site, thus displaces it and drives the progression of cell cycle crossing the G1-S check point. As a result there is compensatory over expression of upstream regulators like p16 [5-9], which inactivate cyclinD-CDK4 and cyclin D-CDK6 complexes trying to retain RB in active hypophosphorylated form.
Thus, persistent infection by high risk HPVs leads confluent nuclear and cytoplasmic positivity of p16 in cells;- otherwise known as block positivity. As the detection of HPVs by hybridization or other molecular methods is quite cumbersome and pretty costly; p16 positivity in cervix gradually emerged as the surrogate marker of high risk HPV infection [10,11].
Galectins are carbohydrate binding proteins having high affinity for beta galactosides. Galectin-3 is the lone member of the chimeric group having a single Carbohydrate Recognition Domain (CRD) with a unique N-terminal domain. The Novel Antiapoptotic Molecule with functional BH1 (NWGR) motif within CRD is important for interactions with various anti-apoptotic B-cell Lymphoma/Leukaemia-2 (BCL2) family proteins [12]. Also it has the unique ability to form pentamers and it allows to form lattices with glycolipids and glycoproteins [12].
Recent studies are focussing on the tumour microenvironment more often after realisation of the fact that the cells of tumour niche may be drivers of tumourogenesis rather than acting as mere bystanders or supportive cells. In this scenario, galectin-3, a ubiquitous agent likely to modulate different pro-survival properties necessary for neoplastic cells like 1) positively regulating survival signalling [13] and suppressing stress pathways [14], 2) blocking immune survillence [15] by inhibition of immune cells [16], 3) modulating cell adhesion [17,18] to regulate contact with stromal cells, promoting metastasis and skewing tumour cells homing to protective niches 4) suppressing tumor cell differentiation 5) controlling endocytosis of critical cell surface receptors 6) regulating cell survival cascades essential for tumour cells to survive changes in oxygen and metabolite content [19-21], is recently emerging as the guardian of tumour microenvironment.
The role of galectin-3 in various cancers is complex [22-29]. The elevated levels have been shown to prognosticate for poor survival in cancers like lymphoma [30], leukaemia [31], breast cancer and thyroid cancer [32] but decreased level appears to be detrimental to patients suffering from chronic lymphoblastic leukaemia and prostate cancer. A possible explanation can involve the intracellular location of the marker [33].
Although association of positive p16 expression with high risk HPV related cases of squamous cell carcinoma of uterine cervix is now a well established fact, there are instances of p16 negative cases also [34]. As p16 is considered as the surrogate marker of HPV infection, this study is expected to re-evaluate its role in progression of cervical SCC, especially the relative contribution of HPV in different morphological variants.
Owing to the emerging role of galectin-3 as the guardian of tumour microenvironment, there are conflicting results [35-43] on its role in cervical tumour biology from different studies, some have documented a positive correlation between its expression and increased tumour invasion [36-41]; while some have demonstrated the opposite [35,37]. Also its differential expression in different morphological variants of SCC has not been studied before.
Objectives
To study the expression of p16 and galectin-3 in different histomorphological variants of cervical squamous cell carcinoma
To study the association of the expression profile of those two markers with tumour grade and stage
Materials and Methods
An observational, descriptive, cross-sectional study was undertaken in the Department of Pathology in a tertiary care Institution of Kolkata from January 2019 to June 2020 (IEC reg no:ECR/322/Inst/WB/2013). Fifty three samples diagnosed as invasive SCC of uterine cervix were taken by systematic random sampling.
Inclusion criteria: Histopathological specimens received in the Department of Pathology within the study period (either received as cervical punch biopsy or surgically excised specimen) morphologically diagnosed as squamous cell carcinoma of uterine cervix were included.
Exclusion criteria: The specimens of benign cervical lesions, cervical intraepithelial neoplasms or squamous in-situ lesions, or of cervical carcinoma morphologically different from squamous cell carcinoma like cervical adenocarcinoma were excluded from the study.
Ultrathin (3-4 microns) sections are obtained by microtomy from the formalin fixed paraffin embedded blocks. After floatation they were picked on poly-L-lysine coated slides, dried, deparaffinized and rehydrated in descending grades of alcohol.
Heat Induced Epitope Retrieval (HIER) procedure was done by microwave method using Tris Hydroxymethyl Aminomethane (TRIS) Buffer, EMPARTA, pH 9.0. TRIS Buffer (EMPARTA, pH 7.2) was used for washing. Endogenous peroxidase activity was blocked with PolyExcel Peroxidase Block, (PATHNSITU) Incubation with primary antibody (Monoclonal antibody against p16-p16 G175405 MonC, PATHNSITU & Monoclonal antibody against galectin-3-Galectin-3-9 MIB, PATHNSITU) was done at 37oC for 60 minutes. For visualisation of result, serial incubation for 30 minutes each was carried out with PolyExcel Target Binder, PATHNSITU; Poly Horse Radish Peroxidase (HRP) (PolyExcel HRP DAB Detection System, PATHNSITU) and chromogen (Polyexcel Stunn DAB Buffer & Polyexcel Stunn Diaminobenzidine (DAB) Chromogen, PATHNSITU).
The sections were then counterstained with Harris Haematoxylin and mounted. Sections of chronic cervicitis were taken as control group. For validation of galectin-3 staining; section of Papillary Thyroid carcinoma was used as positive control.
Proportional average expression of p16 and galectin-3 were allocated by semi quantitative method using light microscopy, based on the overall impression, after scrutinizing the whole slide especially focussing on the hot spot zones. Intensity of the immunostaining were taken as 1+, 2+, 3+ depending upon the positivity. For statistical purposes, 75% proportional positivity of p16 and 50% positivity of galectin-3 in the neoplastic cell population were taken as positive [38].
Statistical Analysis
The data was collected and analysed by Chi-square test and Fisher’s exact test using SPSS, version 25.0.
Results
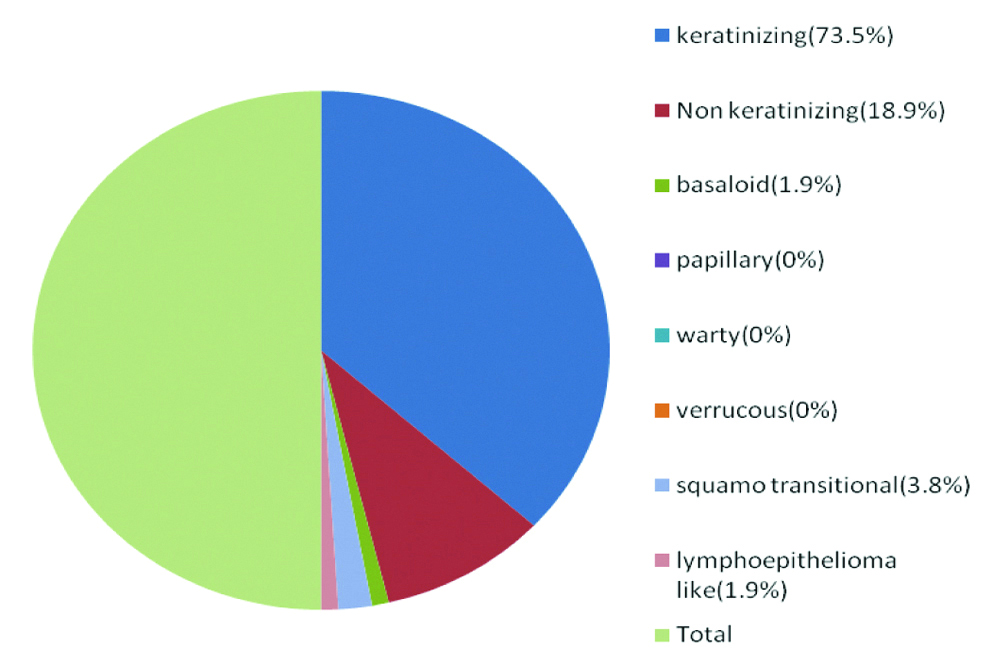
Total of 73.5% (39/53) cases are Keratinizing variant of squamous cell carcinoma (most prevalent type in this study) succeeded by Non-keratinizing variant (10/53) (18.9%). Two cases of squamo transitional (3.8%) and one case each from the Basaloid (1.9%) and Lymphoepithelioma (1.9%) like variant were included in the study. No cases of Papillary, verruccous and warty variants were found within the proposed study duration, so these three variants are not included [Table/Fig-1].
Pie chart depicting relative proportion of cases.
All but one case (which belongs to the keratinizing variant) showed positive expression of p16 [Table/Fig-2].
Distribution of p16 positivity in different variants of cervical squamous cell carcinoma (n=53).
| Histological type | P16 expression | Total |
|---|
| Positive | Negative |
|---|
| Keratinizing | 38 (97.4%) | 1 (2.6%) | 39 (100%) |
| Non keratinizing | 10 (100%) | 0 (0%) | 10 (100%) |
| Basaloid | 1 (100%) | 0 (0%) | 1 (100%) |
| Squamo transitional | 2 (100%) | 0 (0%) | 2 (100%) |
| Lymphoepithelioma like | 1 (100%) | 0 (0%) | 1 (100%) |
| Total | 52 (98.1%) | 1 (1.9%) | 53 (100%) |
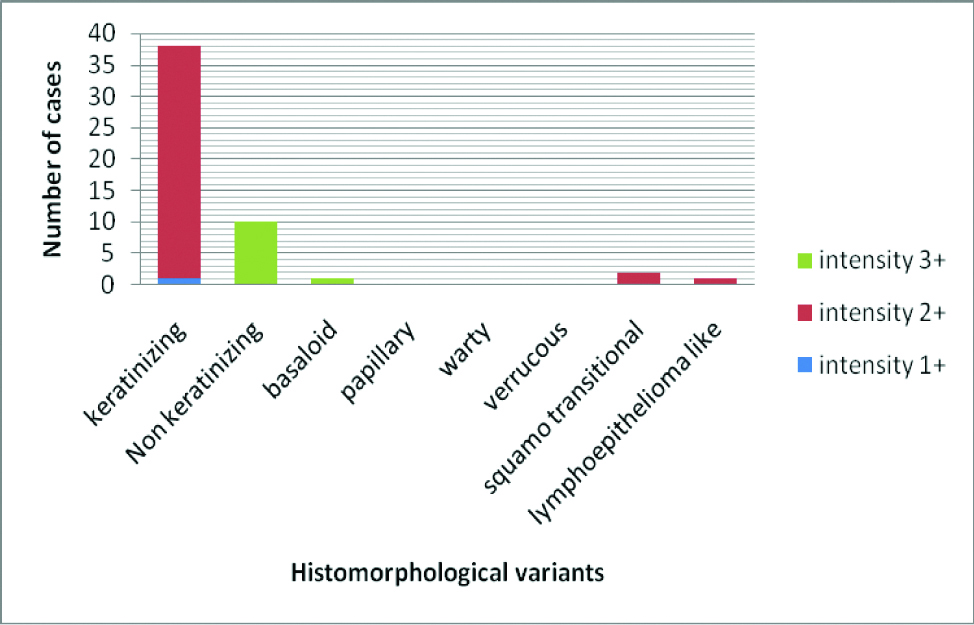
100% of Non-keratinizing and Basaloid variant showed highest (3+) intensity of p16 expression and 97.4% (37/38) of keratinizing variant showed moderate (2+) intensity of p16 staining [Table/Fig-3].
Bar diagram showing distribution of p16 intensity with respect to different histomorphological variants in p16 positive cases.
Galectin was positive in keratinzing, non-keratinizing, squamo transitional and lymphoepithelioma variant. However it showed 100% negativity in basaloid variant [Table/Fig-4].
Distribution of Galectin-3 status among different variants of cervical squamous cell carcinoma (n=53).
| Histological type | Galectin-3 status | Total |
|---|
| Positive | Negative |
|---|
| Keratinizing | 21 (53.8%) | 18 (46.2%) | 39 (100%) |
| Non keratinizing | 1 (10%) | 9 (90%) | 10 (100%) |
| Basaloid | 0 (0%) | 1 (100%) | 1 (100%) |
| Squamo transitional | 2 (100%) | 0 (0%) | 2 (100%) |
| Lymphoepithelioma like | 1 (100%) | 0 (0%) | 1 (100%) |
| Total | 25 (47.2%) | 28 (52.8%) | 53 (100%) |
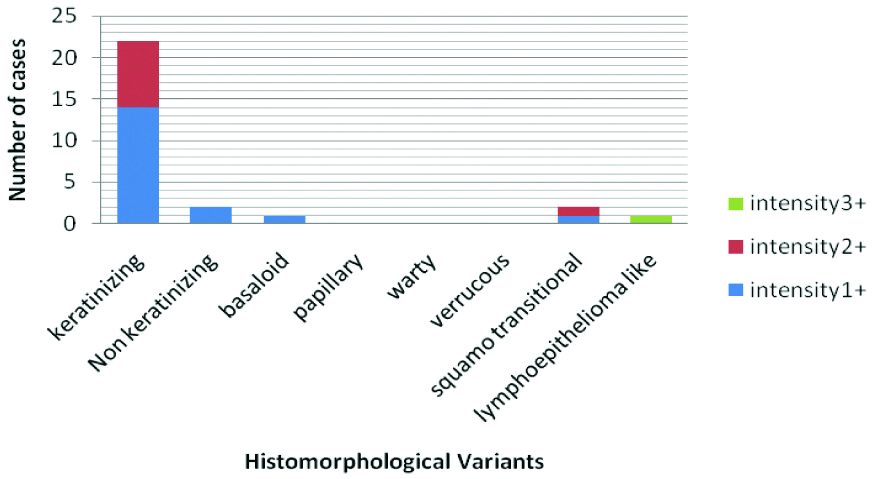
The single case of Lymphoepithelioma like variant showed strongest (3+) intensity of galectin-3 expression. Those cases of non-keratinizing and basaloid variant which were positive for Galectin-3, only showed weak (1+) expression. In 63.6% (14/22) of the cases of keratinizing variant showing galectin-3 positivity, showed 1+intensity; the rest showed moderate (2+) intensity of staining. Among the two cases of squamo-transitional variant, one showed 1+and the other showed 2+intensity of staining [Table/Fig-5].
Bar Diagram showing Disribution of intensity of galectin-3 among different variants of galectin-3 positive squamous cell carcinomas.
Significant association was found between tumour grade and p16 intensity in p16 positive cases. (p=0.0002111) [Table/Fig-6].
Distribution of cases according to Tumour grade and p16 intensity in p16 positive cases (n=52).
| Grade | p16 staining Intensity | Total |
|---|
| 1+ | 2+ | 3+ | |
| Grade 1 | 1 (33.3%) | 2 (66.7%) | 0 (0%) | 3 (100%) |
| Grade 2 | 0 (0%) | 29 (93.5%) | 2 (6.5%) | 31 (100%) |
| Grade 3 | 0 (%) | 9 (50%) | 9 (50%) | 18 (100%) |
| Total | 1 (1.9%) | 40 (76.9%) | 11 (21.2%) | 52 (100%) |
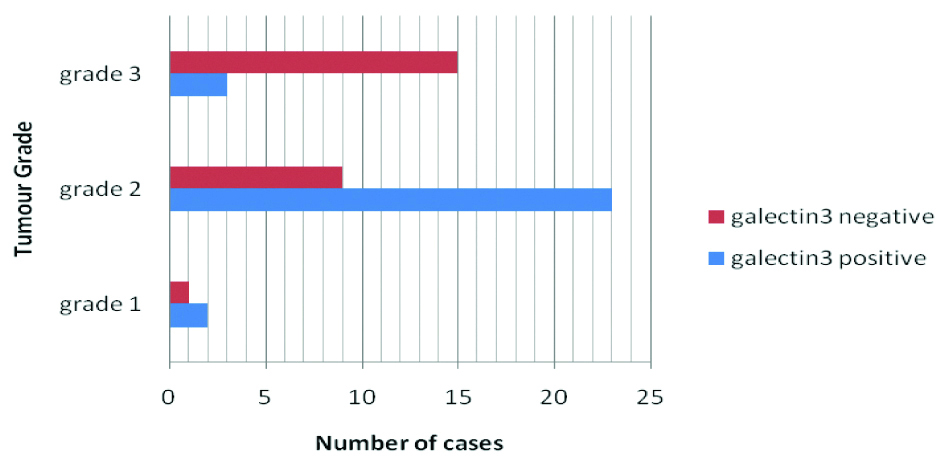
Proportion of galectin-3 positive cases among grade 1, grade 2 and grade 3 tumours are 66.7%, 71.9% and 16.7% respectively [Table/Fig-7]. Statistically significant association was found between galectin-3 positivity and the different histomorphological variants of cervical squamous cell carcinoma (p=0.00448). Although no significant association was found between the positivity (p=0.857344) or intensity of galectin-3 (p=0.211128) and Tumour stage, it becomes apparent that galectin-3 positivity decreases with increasing grade (p=0.000771) [Table/Fig-7].
Bar diagram showing Distribution of cases according to Tumour grade and Galectin 3 status.
Discussion
Among the cases of SCC cervix dealt in the present study, belonging to a wide age range of 32 years to 78 years, Keratinizing subtype [Table/Fig-8] is the predominant variant encountered among all the age groups succeeded by Non Keratinizing subtype [Table/Fig-1]. Majority of these cases (83.0%) were diagnosed in an advanced stage (clinically International Federation of Gynaecology and Obstetrics (FIGO) stage II B or greater, that is cancer with parametrial invasion or higher) and hence rendered as inoperable.
Keratinizing SCC, (H&E, 100x).
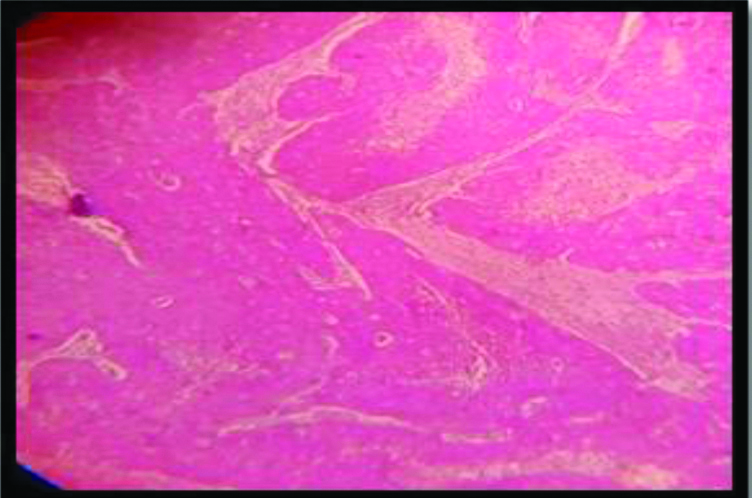
All but one of the 53 cases in this study showed almost 100% block positivity of p16 in the tumour cells -strongly corroborative with high risk HPV infection [Table/Fig-2] but the intensity of staining is variable among different variants. The non-keratinizing and the basaloid variant (100%) showed the strongest intensity of staining (3+) in the present study [Table/Fig-3,9,10], which is consistent with the researches carried out Internationally; focussing on specific histomorphological variants of squamous cell carcinoma, those are most consistently associated with high risk HPV infection [1,42]. Total 97.4% of the Keratinizing variant showed intermediate positivity (2+) [Table/Fig-11]. And also the more differentiated it becomes, less becomes the intensity of p16 staining. Even in the same tumour, the foci of relatively poorly differentiated areas showed more intense staining. And this association between the intensity of staining of p16 with the histomorphological variants of Squamous cell carcinoma in cases showing positive p16 expression is statistically significant (p<0.0001). The squamo columnar junction or the transition zone invariably showed block positivity of p16, which supports our present knowledge regarding the predilection of high risk HPVs towards this region. The squamotransitional variant has also shown 2+intensity of p16 expression [Table/Fig-12].
Non Keratinizing SCC: p16 expression(3+); Inset : H&E of the same (100x).
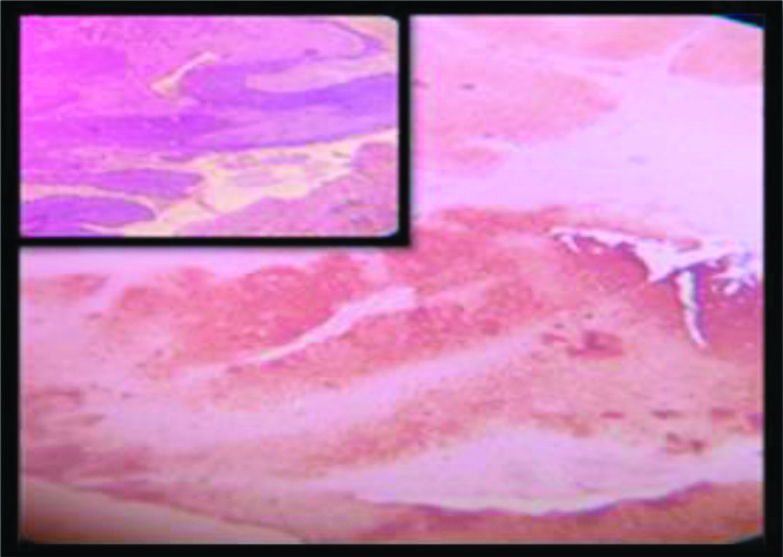
Basaloid SCC: p16 expression(3+); Inset : H&E of the same (100x).
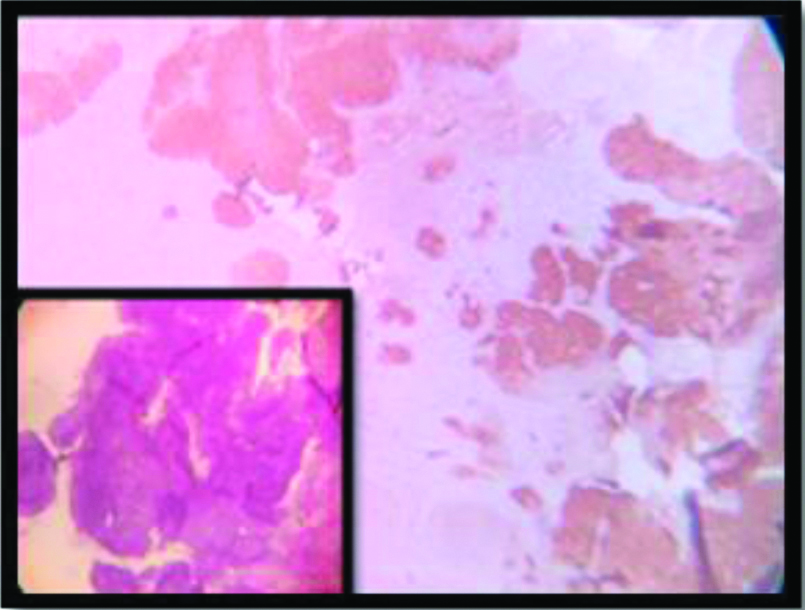
p16 expression (2+) in keratinizing SCC (4x).
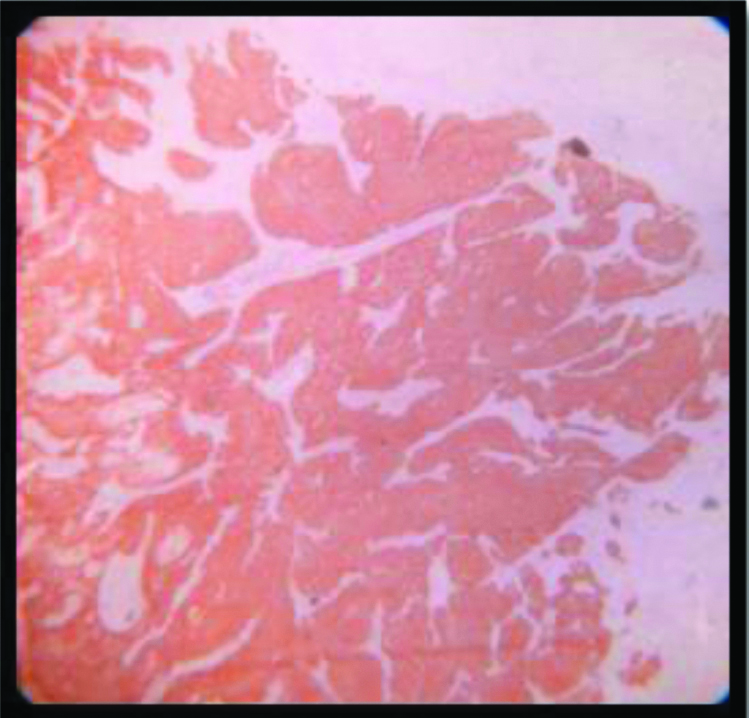
Squamotransitional SCC: p16 expression(2+); Inset: H&E of the same (100x).
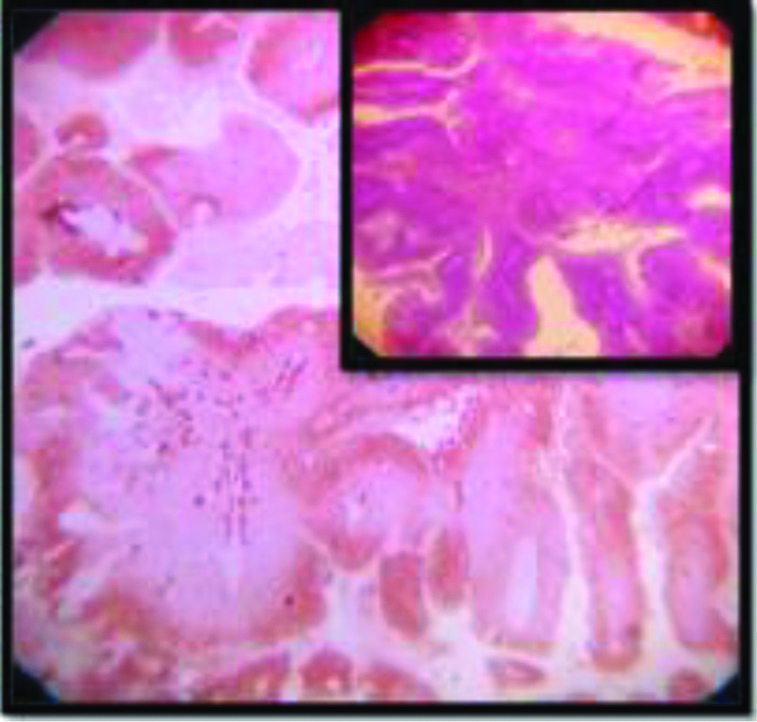
Nuclear grade in case of cervical SCC does not carry much prognostic significance [43]. Same thing can be said for the different histomorphological variants once the clinical stages are matched [43]. However, it is apparent from the present study that Intensity of p16 staining increases with increasing grade (p=0.0002) [Table/Fig-6], which is consistent with our existing knowledge of predilection of high risk HPVs as the causative agent of relatively undifferentiated forms of cervical squamous cell carcinoma [1]. However, no significant association was found between tumour stage and intensity of p16 expression.
Though not very common, there are instances of p16 negative squamous cell carcinomas in literature. The verrucous variant usually shows p16 negativity. Here authors have found a completely p16 negative case of keratinizing squamous cell carcinoma of uterine cervix [Table/Fig-13]. (The authenticity of this finding is proven by the p16 positivity of the squamocolumnar junction in the same slide, which serves as an internal positive control).
p16 negative cervical SCC (H&E:40 X); inset (H&E:4X): squamocolumnar junction.
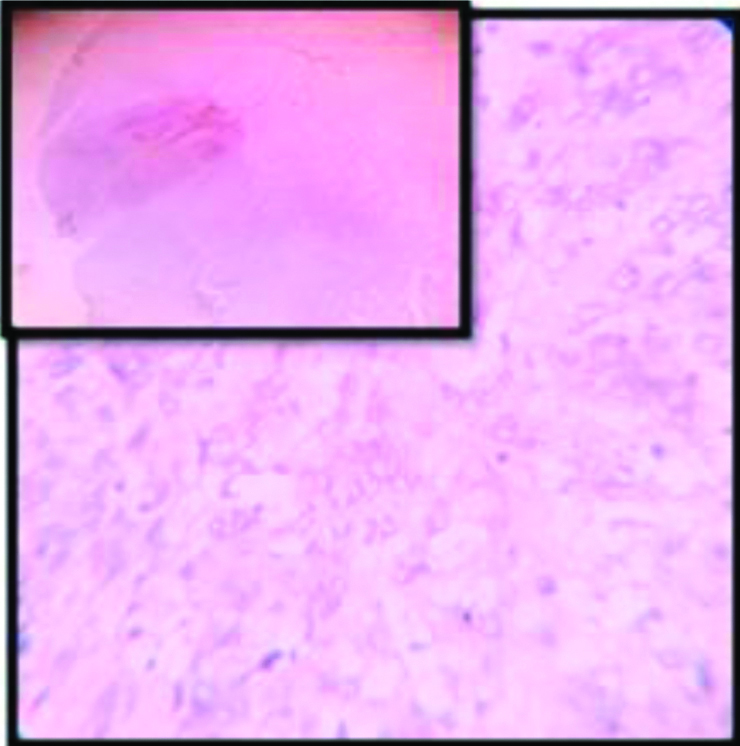
In this case, The p16 positivity of the squamo-columnar junction indicates towards persistent infection of high risk HPV and presumably it was also a key event behind the carcinogenesis as it is commonly seen in other cases. Probably in a late stage of carcinogenesis, a p16 mutation was acquired by the neoplastic clone and therefore the picture we get is that of complete negativity of p16 expression. Similar case of p16 negaive squamous cell carcinoma of cervix is reported by the study conducted by Gupta R et al [44] and Volgareva G et al [45].
Regarding the expression of galectin-3, mostly the tumour cells showed cytoplasmic localisation of this marker in the cases of SCC cervix dealt in this study. The interesting thing is that in galectin-3 positive cases, strong expression of this marker is found in the invasive tongues of the tumour cells at the junction of tumour stromal interface which is consistent with our knowledge of importance of galectin-3 in regulating the tumour microenvironment. This finding is also consistent with the findings described by Punt S et al., [37].
In contray to the findings of Lee JW et al [35], Kim SS et al., [38] concluded that both the positivity of intensity of galectin-3 expression increased as the lesion progresses from intra epithelial lesions to invasive cancer. However, Punt S et al., found that weak and positive galectin-3 expression in tumour cells were correlated with increased and decreased tumour invasion respectively [37].
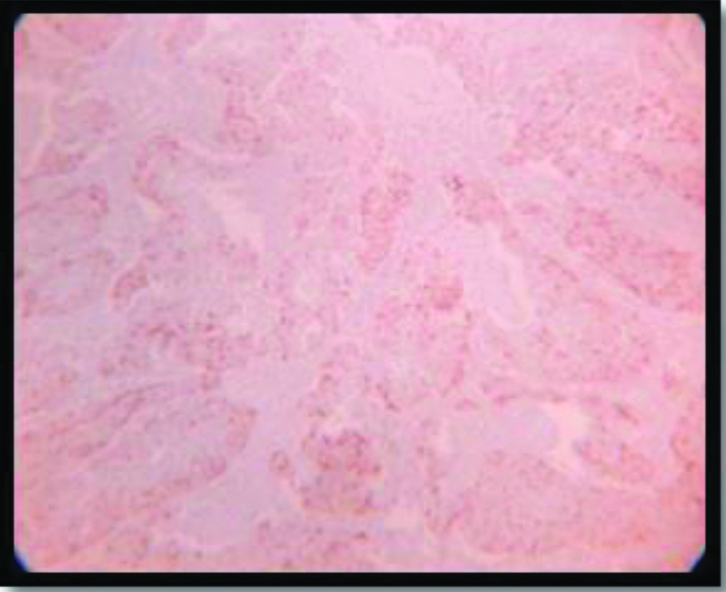
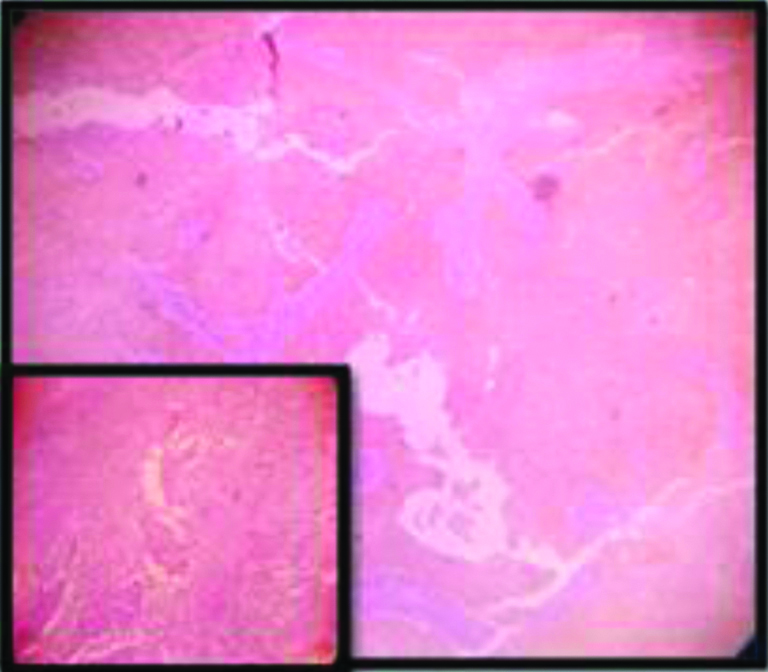
Based on the 50% cut off criteria, 47.2% of total cases were positive for galectin-3 expression. Mostly the keratinized variants (53.8%) showed positivity of galectin-3 [Table/Fig-14] where as the non-keratinizing and Basaloid variant showed almost negative expression (90% of cases) [Table/Fig-4]. The strongest galectin-3 positivity (3+) was found in the single case of Lymphoepithelioma like squamous cell carcinoma and showed almost 100% positivity among the neoplastic cell population [Table/Fig-5,15]. And thus this usually suggests tumour galectin-3 expression may be necessary for a differentiated phenotype; which again corroborates with the findings from the study of Punt S et al [37]. The squamotransitional variant has also shown 2+intensity of galectin-3 expression [Table/Fig-16].
Galectin-3 expression(2+) in Keratinizing SCC (4x).
Lymphoepithelioma like SCC: galectin-3 expression(3+) (10x); Inset: H&E of the same (10x).
Squamotransitional SCC: galectin-3 expression(2+) (10x).
Over all it comes forth that the more poorly differentiated variants like Non-Keratinizing and Basaloid subtype have a propensity to show strong expression of p16 and negative or weak expression of Galectin-3.
Probably it will be best to conclude as it was inferred from the study of Punt S et al., [37] - owing to the diverse and complex actions of galectin-3 in regulating almost all the hallmarks of cancer, it is difficult to draw any direct correlation regarding its expression and the behaviour of invasive cervical cancer. So, in this scenario, it will be challenging to use this marker as a tool of diagnostic or prognostic utility.
Limitation(s)
No case of Papillary, Verrucous and warty variant of cervical squamous cell carcinoma was found within the stipulated time period. So, no inference can be drawn regarding the behaviour of those histomorphological variants from this study.
Conclusion(s)
The basaloid and non-keratinizing variant of squamous cell carcinoma show highest intensity of p16 staining consistent and is associated with high risk HPV infection. Though, most of the cases of cervical squamous cell carcinoma show block positivity of p16 and are associated with high risk HPV infection; cases of p16 negative cervical SCC also exist. Statistically significant association is found between histomorphological variant of SCC cervix and galectin-3 expression as well as tumour grade and galectin-3 positivity with decrease in staining in relation to higher grade; consistent with the assumption that galectin-3 positivity is necessary for a differentiated phenotype. However, the association between tumour stage and expression of this marker was not significant in the present study.
[1]. Kurman RJ, Carcangiu ML, Herrington CS, Young RH, WHO classification of tumours of female reproductive organs 2014 4th editionInternational agency for research on cancer [Google Scholar]
[2]. Bzhalava D, Eklund C, Dillner J, International standardization and classification of human papillomavirus typesVirology 2015 476:341-344.10.1016/j.virol.2014.12.02825577151 [Google Scholar] [CrossRef] [PubMed]
[3]. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Human papillomavirus is a necessary cause of invasive cervical cancer worldwideJ Pathol 1999 189(1):12-49.10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431<3.0.CO;2-F [Google Scholar] [CrossRef]
[4]. Robbins and Cortan. Pathologic basis of disease South Asia Edition, Nineth Edition. 2016 [Google Scholar]
[5]. Moody CA, Human papillomavirus oncoproteins: Pathways to transformationNat Rev Cancer 2010 10(8):55010.1038/nrc288620592731 [Google Scholar] [CrossRef] [PubMed]
[6]. Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T, Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesionsAm J Pathol 1998 153(6):1741-8.10.1016/S0002-9440(10)65689-1 [Google Scholar] [CrossRef]
[7]. Nakao Y, Yang X, Yokoyama M, Ferenczy A, Tang SC, Pater MM, Induction of p16 during immortalization by HPV 16 and 18 and not during malignant transformationBr J Cancer 1997 75(10):1410-16.10.1038/bjc.1997.2439166931 [Google Scholar] [CrossRef] [PubMed]
[8]. Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteriInt J Cancer 2001 92(2):276-84.10.1002/ijc.117411291057 [Google Scholar] [CrossRef] [PubMed]
[9]. Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasiaAm J Surg Pathol 2001 25(7):884-91.10.1097/00000478-200107000-0000611420459 [Google Scholar] [CrossRef] [PubMed]
[10]. Darragh TM, Colgan TJ, Cox T, The lower anogenital squamous terminology standardization project for HPV-associated lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical PathologyInt J Gynecol Pathol 2013 32(1):76-115.10.1097/PGP.0b013e31826916c723202792 [Google Scholar] [CrossRef] [PubMed]
[11]. Klaes R, Benner A, Friedrich T, Ridder R, Herrington S, Jenkins D, p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasiaAm J Surg Pathol 2002 11:1389-99.10.1097/00000478-200211000-0000112409715 [Google Scholar] [CrossRef] [PubMed]
[12]. Ruvolo Peter P, Galectin 3 as the guardian of tumour microenvironmentBiochemica et Biophysica Acta 2019 1863(3):427-37.10.1016/j.bbamcr.2015.08.00826264495 [Google Scholar] [CrossRef] [PubMed]
[13]. Thijssen VL, Rabinovich GA, Griffioen AW, Vascular galectins: Regulators of tumor progression and targets for cancer therapyCytokine Growth Factor Rev 2013 24:547-58.10.1016/j.cytogfr.2013.07.00323942184 [Google Scholar] [CrossRef] [PubMed]
[14]. Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Galectins and their ligands: Amplifiers, silencers or tuners of the inflammatory response?Trends Immunol 2002 23:313-20.10.1016/S1471-4906(02)02232-9 [Google Scholar] [CrossRef]
[15]. Novak R, Dabelic S, Dumic J, Galectin-1 and galectin-3 expression profiles in classically and alternatively activated human macrophagesBiochim Biophys Acta 2012 1820:1383-90.10.1016/j.bbagen.2011.11.01422155450 [Google Scholar] [CrossRef] [PubMed]
[16]. Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell deathJ Immunol 2006 176:778-89.10.4049/jimmunol.176.2.77816393961 [Google Scholar] [CrossRef] [PubMed]
[17]. Inohara H, Akahani S, Koths K, Raz A, Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesionCancer Res 1996 56(19):4530-34. [Google Scholar]
[18]. Inohara H, Raz A, Functional evidence that cell surface galectin-3 mediates homotypic cell adhesionCancer Res 1995 55:3267-71. [Google Scholar]
[19]. Markowska AI, Liu FT, Panjwani N, Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic responseJ Exp Med 2010 207:1981-93.10.1084/jem.2009012120713592 [Google Scholar] [CrossRef] [PubMed]
[20]. Funasaka T, Raz A, Nangia-Makker P, Galectin-3 in angiogenesis and metastasisGlycobiology 2014 24(10):886-91.10.1093/glycob/cwu08625138305 [Google Scholar] [CrossRef] [PubMed]
[21]. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastasesCancer Res 1999 59(22):5830-35. [Google Scholar]
[22]. Balan V, Nangia-Makker P, Raz A, Galectins as cancer biomarkersCancers (Basel) 2010 2:592-610.10.3390/cancers202059223658855 [Google Scholar] [CrossRef] [PubMed]
[23]. Righi A, Morandi L, Leonardi E, Farnedi A, Marucci G, Sisto A, Galectin-3 expression in pituitary adenomas as a marker of aggressive behaviorHum Pathol 2013 44:2400-09.10.1016/j.humpath.2013.05.02024007691 [Google Scholar] [CrossRef] [PubMed]
[24]. Zhou X, Jing J, Mao W, Zheng Y, Wang D, Wang X, Expression and clinical significance of galectin-3 in osteosarcomaGene 2014 546:403-07.10.1016/j.gene.2014.04.06624786210 [Google Scholar] [CrossRef] [PubMed]
[25]. Thijssen VL, Heusschen R, Caers J, Griffioen AW, Galectin expression in cancer diagnosis and prognosis: A systematic reviewBiochim Biophys Acta 2015 1855:235-47.10.1016/j.bbcan.2015.03.00325819524 [Google Scholar] [CrossRef] [PubMed]
[26]. Barrow H, Rhodes JM, Yu LG, The role of galectins in colorectal cancer progressionInt J Cancer 2011 129(1):01-08.10.1002/ijc.2594521520033 [Google Scholar] [CrossRef] [PubMed]
[27]. Zaia Povegliano L, Oshima CT, de Oliveira Lima F, Andrade Scherholz PL, Manoukian Forones N, Immunoexpression of galectin-3 in colorectal cancer and its relationship with survivalJ Gastrointest Cancer 2011 42(4):217-21.10.1007/s12029-010-9189-120635166 [Google Scholar] [CrossRef] [PubMed]
[28]. Gomes TS, Oshima CT, Forones NM, De Oliveira LF, Ribeiro DA, Expression of galectin-3 in gastric adenocarcinomaIndian J Med Res 2014 140(1):69-76. [Google Scholar]
[29]. Tsuboi K, Shimura T, Masuda N, Ide M, Tsutsumi S, Yamaguchi S, Galectin-3 expression in colorectal cancer: Relation to invasion and metastasisAnticancer Res 2007 27(4B):2289-96. [Google Scholar]
[30]. Konstantinov KN, Robbins BA, Liu FT, Galectin-3, a beta-galactoside- binding animal lectin, is a marker of anaplastic large cell lymphomaAm J Pathol 1996 148:25-30. [Google Scholar]
[31]. St-Pierre Y, Galectins in hematological malignanciesAm J Blood Res 2011 1(12):119-29. [Google Scholar]
[32]. Chiu CG, Strugnell SS, Griffith OL, Jones SJ, Gown AM, Walker B, Diagnostic utility of galectin-3 in thyroid cancerAm J Pathol 2010 176(5):2067-81.10.2353/ajpath.2010.09035320363921 [Google Scholar] [CrossRef] [PubMed]
[33]. Haudek KC, Spronk KJ, Voss PG, Patterson RJ, Wang JL, Arnoys EJ, Dynamics of galectin-3 in the nucleus and cytoplasmBiochim Biophys Acta 2010 1800:181-89.10.1016/j.bbagen.2009.07.00519616076 [Google Scholar] [CrossRef] [PubMed]
[34]. Casey S, Harley I, Jamison J, A rare case of HPV-negative cervical squamous cell carcinomaInt J Gynecol Pathol 2015 34(2):208-12.10.1097/PGP.000000000000013225675193 [Google Scholar] [CrossRef] [PubMed]
[35]. Lee JW, Song SY, Choi JJ, Choi CH, Kim TJ, Kim J, Decreased galectin-3 expression during the progression of cervical neoplasiaJ Cancer Res Clin Oncol 2006 132(4):241-47.10.1007/s00432-005-0069-116369807 [Google Scholar] [CrossRef] [PubMed]
[36]. Liu J, Cheng Y, He M, Yao S, Vascular endothelial growth factor C enhances cervical cancer cell invasiveness via upregulation of galectin-3 proteinGynecol Endocrinol 2014 30(6):461-65.10.3109/09513590.2014.89805424650367 [Google Scholar] [CrossRef] [PubMed]
[37]. Punt S, Victor LT, Johannes V, Cornelis DK, Arko G, Ekaterina SJ, Galectin 1, 3 and 9 expression and clinical significance in squamous Cervical cancerPLoS ONE 2015 10(6)10.1371/journal.pone.012911926066796 [Google Scholar] [CrossRef] [PubMed]
[38]. Kim SS, Cho HY, Kang SW, Kim HB, Park SH, Is the expression of p16ink4A and Galectin 3 correlated with disease progression of cervical neoplasia?Korean J Obstet Gynecol 2011 54(4):192-98. [Google Scholar]
[39]. Jianting Ma, Xingguang Zhang, Gang He, Chunlin Yang, The relationship between cervical precancerous lesion galectin 3 and p27 protein expression and clinical prognosisOncology Letters 2017 15(2):1533-36.10.5468/KJOG.2011.54.4.192 [Google Scholar] [CrossRef]
[40]. Li M, Feng YM, Feng SQ, Overexpression of ezrin and galectin 3 as predictors of poor prognosis of cervical cancerBraz J Med Biol Res 2017 50(4):e535610.1590/1414-431x2016535628355349 [Google Scholar] [CrossRef] [PubMed]
[41]. Annika Stiasny, Christoph P Freier, Christina Kuhn, Bernd P Kost, The involvement of E6, p53, p16, MDM2 and Galectin 3 in the clinical outcome of patients with cervical cancerOncology Letters 2017 14(4):4467-76.10.3892/ol.2017.675229085443 [Google Scholar] [CrossRef] [PubMed]
[42]. Kurman RJ, Toki T, Schiffman MH, Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomavirusesAm J Surg Pathol 1993 17(2):133-45.10.1097/00000478-199302000-000058380681 [Google Scholar] [CrossRef] [PubMed]
[43]. Rosai and Ackermann. Surgical Pathology. Eleventh Edition, Elsevier. 2018 [Google Scholar]
[44]. Gupta R, Srinivasan R, Nijhawan R, Suri V, Uppal R, Protein p16 INK4A expression in cervical intraepithelial neoplasia and invasive squamous cell carcinoma of uterine cervixIndian J Pathol Microbiol 2010 53(1):07-11.10.4103/0377-4929.5917420090213 [Google Scholar] [CrossRef] [PubMed]
[45]. Volgareva G, Zavalishina L, Andreeva Y, Frank G, Krutikova E, Goloniva D, Protein p16 as a marker of dysplastic and neoplastic alterations in cervical epithelial cellsBMC cancer 2004 4:5810.1186/1471-2407-4-5815339339 [Google Scholar] [CrossRef] [PubMed]