Pulmonary Lymphangioleiomyomatosis (LAM) is a rare disease, mostly seen in women of reproductive age group. Most common presentation is dyspnoea on exertion, may be associated with haemoptysis and chest pain. LAM is a cystic lung disease, with a poor prognosis and is difficult to treat. In this case report, two patients are discussed who presented with dyspnoea and chest pain due to pneumothorax and were later diagnosed as case of LAM on clinical and radiological basis. Intercostal Chest Tube Drainage (ICD) was inserted for resolution of pneumothorax and subsequently pleurodesis was done to prevent recurrent pneumothorax. In case of recurrent pneumothorax, LAM should be considered as a differential diagnosis. Pleurodesis can be considered to prevent recurrent pneumothorax.
Case Presentation
Case 1
A 26-year-old married female, presented in Respiratory Medicine OPD with complaints of right-sided chest pain for last one year which was intermittent, pleuritic in nature, non-radiating, increased in right lateral position, relieved on sitting and bending forward position. She also had complaints of dry cough and breathlessness, on and off for last one year, which increased in severity for two months. Patient had similar complaints one year before for which she visited local practitioner and was treated symptomatically with bronchodilator and oxygen inhalation. Patient had no history of diabetes, hypertension and tuberculosis. Family history was not significant. On general examination- Patient had pulse rate-116/min, BP-110/70 mmHg, respiratory rate-28/min, saturation-95% on room air.
In respiratory system examination, inspection showed right-sided chest bulge and decreased chest movements on same side. Inspection findings were confirmed by palpation. On percussion, hyper-resonant note was noted in right hemithorax. On auscultation, decreased breath sound intensity and decreased vocal resonance was found in right hemithorax.
The differential diagnoses of pneumothorax, Chronic Obstructive Pulmonary Disease (COPD), Langerhans Cell Histiocytosis (LCH) and LAM was made initially on the basis of history and clinical examination. For confirmation of diagnosis, radiological evaluation was made.
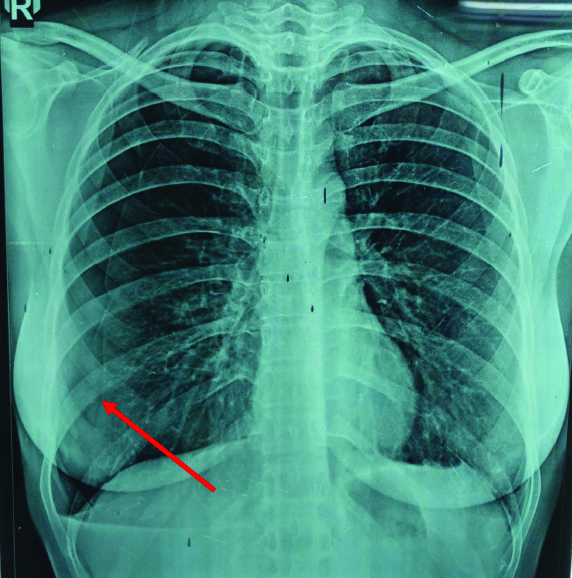
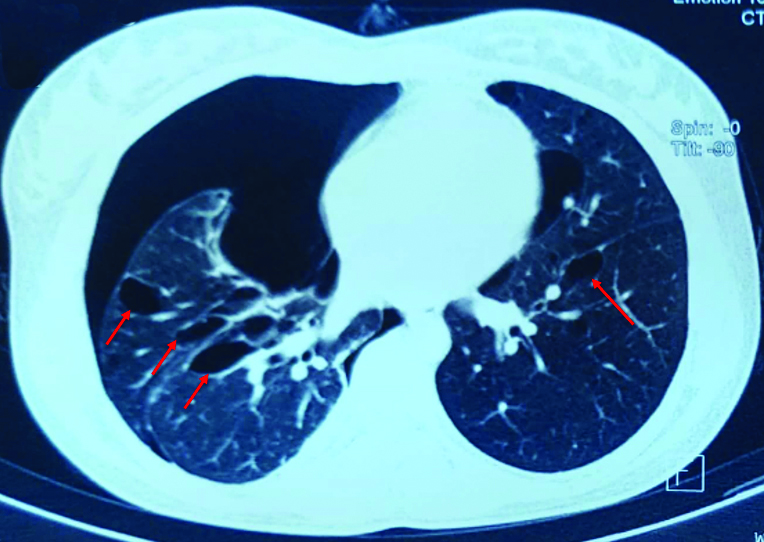
Chest x-ray was suggestive of right-sided pneumothorax [Table/Fig-1] and HRCT thorax [Table/Fig-2] revealed bilateral multiple emphysematous bullae with multiple thin walled cysts in lung parenchyma, large right-sided pneumothorax, small pneumothorax in left apical region, atelectatic strands in right upper lobe and right lower lobe and few calcified lymph nodes in subcarinal and right hilar region. After complete clinical and radiological evaluation (numerous thin wall cysts with normal intervening lung on HRCT), patient was diagnosed as a case of LAM.
CXR-PA view showing right-sided pneumothorax (Red arrow showing visceral pleural line). CXR: Chest x-ray; PA: Posteroanterior
CT Thorax showing bilateral multiple cysts in lung parenchyma (Red arrows).
Further patient was treated conservatively with antibiotic (doxycycline) and chest tube was inserted on right side for resolution of pneumothorax. Later, talc pleurodesis was done to prevent recurrence of pneumothorax. Patient was discharged after stabilisation with minimal symptoms of dyspnoea, for which formoterol (6 mcg) with budesonide (200 mcg) inhaler with spacer (2 puffs twice daily) was prescribed. Patient showed symptomatic improvement on regular monthly follow-up. No complications or recurrence of pneumothorax encountered upto 12 months follow-up.
Case 2
A 34-year-old male, non-smoker presented to our respiratory medicine OPD with chief complaints of dyspnoea and chest pain for last three days. Chest pain was intermittent, right-sided, sudden in onset, pleuritic and non-radiating. He had similar complaints two months back and was diagnosed as a case of pneumothorax for which ICD was placed. Pneumothorax was resolved. Patient had no past history of tuberculosis, hypertension, diabetes or any other chronic illness. No family history of any similar illness was present. On general examination- Patient’s vitals were- pulse rate-110/min, BP-124/76 mmHg, respiratory rate-30/min, saturation-94% on room air. Respiratory system examination revealed decreased chest movements on right side on inspection, hyper-resonant note in right hemithorax on percussion and decreased breath sound intensity auscultated in right hemithorax.
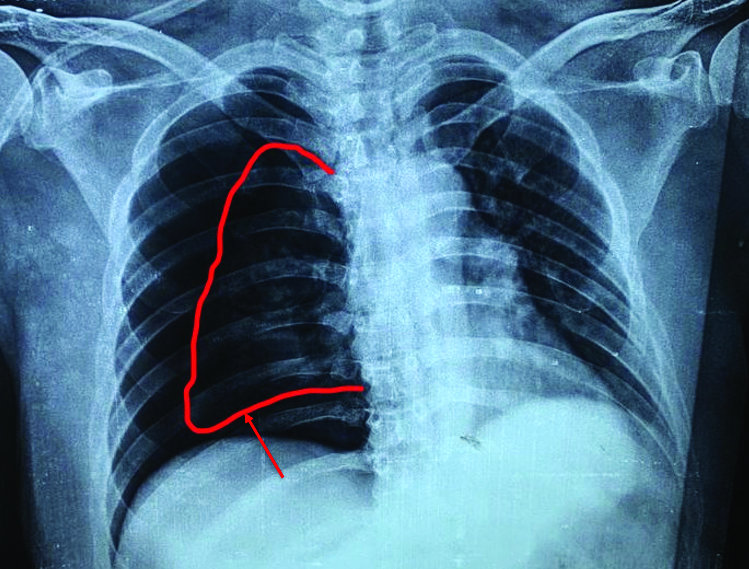
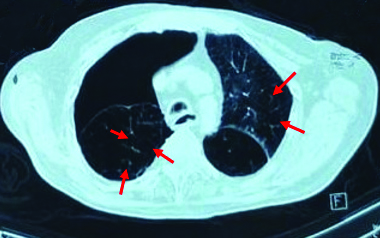
The differential diagnoses of pneumothorax, COPD, LCH and LAM was considered based on history and clinical examination. For confirmation of diagnosis, radiological evaluation was done. Chest x-ray revealed right-sided pneumothorax [Table/Fig-3]. To find the cause of recurrent pneumothorax, CT thorax was done. It suggested large right-sided pneumothorax with multiple thin walled cysts in both lungs [Table/Fig-4]. Based on CT report and clinical features, diagnosis of LAM was made.
CXR s/o right-sided pneumothorax. (Red arrow showing visceral pleural line).
HRCT Thorax showing bilateral multiple cysts in lung parenchyma (Red arrows) and right-sided pneumothorax (Red arrow).
Patient was treated with oxygen inhalation and ICD was inserted on right side to resolve pneumothorax followed by talc pleurodesis to prevent its recurrence. He was discharged on bronchodilator, in form of Metered Dose Inhaler (MDI) with spacer, for minimal symptom of dyspnoea. On regular follow-up, he showed symptomatic improvement and no recurrence of pneumothorax upto 12 months.
Discussion
Lymphangioleiomyomatosis is a rare group of disorder commonly seen in females of reproductive age group. It is characterised by abnormal proliferation of smooth muscle cells [1], most notably in the lung parenchyma and airway walls, as well as the lymphatics. As the disease progress, it leads to narrowing and obstruction of the airway that presents as obstructive lung disease. Later leads to alveolar damage and development of cystic disease of lungs [2]. Patients with severe LAM with Diffusion Lung Capacity of Carbon Monoxide (DLCO) and/or Forced Expiratory Volume in 1st second (FEV1) <40% of predicted value and require continuous oxygen therapy should be referred for lung transplantation [3]. The estimated prevalence of LAM is thought to be around 2.6 per 10,00,000 in general female population [4].
The clinical appearance of LAM very much varies, but most common appearance is dyspnoea on exertion [2,5]. Other frequent physical findings of LAM include spontaneous pneumothorax (57%), haemoptysis (32%), abdominal angiolipoma (32%), lymphangioleiomyoma (29%) and pleural effusion (12%), and less commonly chylothorax, chylous ascites, chyluria and chyloptysis [2]. In this case, patient presented with dyspnoea and chest pain, and later diagnosed to have pneumothorax.
Initial presentation is like obstructive airway disease. Thus, often misdiagnosed and treated with bronchodilators. Spontaneous pneumothorax occurs due to proliferation of smooth muscles in the bronchioles. Air trapping occurs due to airway narrowing, thus, leading to multiple cysts formation. One study reported 40-80% of LAM patients have recurrent pneumothorax [6]. CT Scan shows diffuse cystic lung parenchyma.
Rhee JA et al., reported a similar case of 55-year-old female who presented with right-sided chest pain and aggravated breathlessness since three days. Chest x-ray showed right-sided pneumothorax with small pleural effusion. Chest tube was inserted for pneumothorax. Subsequent CT thorax revealed bilateral multiple cysts [2]. In this case, patient presented with similar complaints and CT thorax showed right-sided pneumothorax with bilateral multiple thin walled cysts; a possibility of LAM. Chest tube was inserted on right side for resolution of pneumothorax; followed by talc pleurodesis to prevent recurrent pneumothorax.
Treatment is aimed to treat complications. Bronchodilators are part of supportive measure in LAM patients with dyspnoea [2]. Some patients were started on sirolimus or everolimus, immune-modulating therapy that target mammalian Target Of Rapamycin (mTOR) signaling pathway, by inhibiting the mTOR complex which provides a mean transplant free survival of approx. 29 years from onset of symptoms and 10-year transplant free survival of 86% [7]. But these are only stabilising agents and not the curative one. Lung transplantation remains the last treatment option for patients with advanced LAM. In the present case, patient was treated for pneumothorax by inserting ICD and given bronchodilators for dyspnoea.
LAM in males is a very rarely reported phenomenon. The one who is reported in AJRCCM by Aubry MC et al., was a patient of Tuberous Sclerosis Complex (TSC), in which incidence of LAM is 1-3% [8]. Current patient was a phenotypic male with no history of infertility or genital abnormalities, with no features of TSC complex but still presented with LAM features, as against literature which suggests that males who are either female genotypically or associated with TSC complex are found with LAM [8].
When LAM is encountered in male patient, the role of oestrogen and progesterone is questionable in pathogenesis of LAM. Therapeutic strategies have targeted modulation of oestrogen and progesterone levels. Indeed, a trial of medroxyprogesterone acetate is the currently recommended treatment of choice [9,10]. In both the cases discussed here, complete resolution of pneumothorax and no recurrence was reported on regular follow-up upto one year. However, due to affordability issues of patients, we were unable to perform biopsies for final results and both the cases were treated on clinico-radiological basis.
Conclusion(s)
LAM is a rare disease which occurs predominantly in young women, but it could occur in males also. It should be considered as differential diagnoses in case of spontaneous pneumothorax, cystic lung disease and COPD. Early diagnosis and prevention is the primary problem. CT thorax can be used as an early diagnostic modality. Pleurodesis could be the part of management to prevent recurrence of pneumothorax, which gives better prognostic values.
[1]. Sathirareuangchai S, Shimizu D, Vierkoetter KR, Pulmonary Lymphangio leiomyomatosis: A case report and literature reviewHawaii J Health Soc Welf 2020 79(7):224-29.PMID: 32666056; PMCID: PMC7350511 [Google Scholar]
[2]. Rhee JA, Adial A, Gumpeni R, Iftikhar A, Lymphangioleiomyomatosis: A case report and review of literatureCureus 2019 11(1):e393810.7759/cureus.393830937236 [Google Scholar] [CrossRef] [PubMed]
[3]. Taveira-Dasilva AM, Steagall WK, Moss J, LymphangioleiomyomatosisCancer Control 2006 :276-85.10.1177/10732748060130040517075565 [Google Scholar] [CrossRef] [PubMed]
[4]. Yamazaki A, Miyamoto H, Futagawa T, Oh W, Sonobe S, Takahashi N, An early case of pulmonary lymphangioleiomyomatosis diagnosed by video-assisted thoracoscopic surgeryAnn Thorac Cardiovasc Surg 2005 11(6):405-07.PMID: 16401991 [Google Scholar]
[5]. Zhang X, Travis WD, Pulmonary lymphangioleiomyomatosisArch Pathol Lab Med 2010 134(12):1823-28.10.5858/2009-0576-RS.121128782 [Google Scholar] [CrossRef] [PubMed]
[6]. Carrington CB, Cugell DW, Gaensler EA, Marks A, Redding RA, Schaaf JT, Lymphangioleiomyomatosis. Physiologic-pathologic-radiologic correlationsAm Rev Respir Dis 1977 116(6):977-95.doi: 10.1164/arrd.1977.116.6.977. PMID: 931190 [Google Scholar]
[7]. Oprescu N, McCormack FX, Byrnes S, Kinder BW, Clinical predictors of mortality and cause of death in lymphangioleiomyomatosis: A population-based registryLung 2013 191(1):35-42.Epub 2012 Sep 2510.1007/s00408-012-9419-323007140 [Google Scholar] [CrossRef] [PubMed]
[8]. Aubry MC, Myers JL, Ryu JH, Henske EP, Logginidou H, Jalal SM, Pulmonary lymphangioleiomyomatosis in a manAmerican Journal of Respiratory and Critical Care Medicine 2000 162(2):749-52.10.1164/ajrccm.162.2.991100610934115 [Google Scholar] [CrossRef] [PubMed]
[9]. Taylor JR, Ryu J, Colby TV, Raffin TA, Lymphangioleiomyomatosis. Clinical course in 32 patientsN Engl J Med 1990 323(18):1254-60.10.1056/NEJM1990110132318072215609 [Google Scholar] [CrossRef] [PubMed]
[10]. Johnson S, Rare diseases. 1 Lymphangioleiomyomatosis: Clinical features, management and basic mechanismsThorax 1999 54(3):254-64.10.1136/thx.54.3.25410325903 [Google Scholar] [CrossRef] [PubMed]