Introduction
A group of patients with pneumonia due to an unidentified cause, epidemiologically related to a live food market in Wuhan city of China were reported [1]. World Health Organisation (WHO) labelled the new coronavirus disease as a “pandemic” because of the growing number of cases reported from various nations. This was to tell nations to join their hands to combat this health emergency [2]. Epidemiologists believe that only a vaccine can curb this COVID-19 pandemic. Edward Jenner (Father of Immunology) coined the term ‘vaccine’ from the word Variolae Vaccinae (also known as cowpox). Maurice Hilleman developed 40 vaccines and eight of his vaccines are still recommended in the current American immunisation schedule [3]. Smallpox got eradicated via a vaccine and polio is on the verge of eradication. So, history tells us that vaccines are the most cost-effective tool to conquer infections. Mumps vaccine was developed in just four years [4]. Currently, scientists are racing against time to develop a COVID-19 vaccine quickly. Development of the COVID-19 vaccine is a challenge because even if we make an effective vaccine, distribution and administration to the most vulnerable people in a short interval of time is difficult. Developing countries face extraordinary challenges to maintain a cold chain required for many vaccines. The science of COVID-19 vaccine development has expanded in the past year. For a busy practicing physician, it is important to have a cumulative view of the entire COVID-19 development without getting overloaded by the extensive amount of specialised information available. This review outlines all the information about the coronavirus vaccine, the timeline of developing a vaccine, its platform, the latest result of various clinical trials, challenges faced, and the distribution process. It also throws light on concepts of herd immunity, antibody-dependent enhancement phenomenon, vaccine hesitancy, and steps to improve uptake of COVID-19 shots in developing countries. We aim to provide an integrated, synthesised overview of the current state of the narrative of the COVID-19 vaccine.
Literature Search
A search was conducted on the following electronic databases: PubMed, Google scholar, and WHO COVID-19 database. Also, official webpages from the United States, National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), and the vaccine confidence project, were included as references. The search strategy consisted of the following keywords: “Coronavirus vaccine”, “COVID-19 vaccine”, “SARS-COV-2 vaccine”, “structure”, “risk-factors”, “clinical trial”, “immunisation”, “herd immunity”, “vaccine hesitancy”, “vaccine-enhanced disease”. To avoid missing any eligible studies, all the titles as well as abstracts that emerged from this exploration were reviewed completely. Given the rapidly evolving subject matter of this review, consideration was also given to papers in the pre-publication process. The authors gathered a total of 250 references and analysed them. Ultimately, 54 of these references were included after the removal of duplicate articles. Publications in languages other than English were excluded. Also, a few articles talked about other coronaviruses like the SARS virus and the MERS virus; so they were filtered out.
Structure
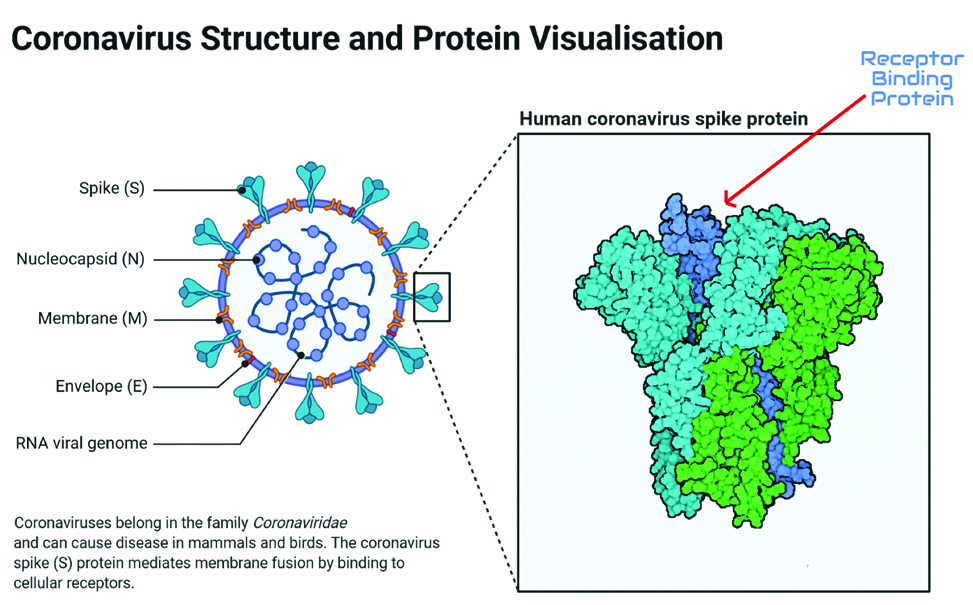
Coronavirus looks like a crown under an electron-microscope because of its external protein protrusions, hence the name. They are pleomorphic and enveloped. The genome of the virus is single-stranded positive-sense RNA. Structural proteins are M (membrane), S (spike), E (envelope), and N (nucleocapsid) [Table/Fig-1]. S glycoprotein spikes present on the external surface help SARS-CoV-2 to attach to the Angiotensin-Converting Enzyme (ACE 2) receptor, fuse, and enter into the human cell. Once inside the cell, coronavirus releases its RNA into the cytosol of human cells [5,6].
Coronavirus structure with its four protein and RNA genome is shown. The receptor-binding domain (shown by red arrow) of S protein will be targeted by neutralising antibodies. Image created using Biorender.com.
Vaccine Development Timeframe
Developing vaccines usually takes a long time. Versions of the vaccine are tested in iterative processes; the constructs are optimised and this can take a few years. Then, a commercial partner needs to be searched who can fund advancement into clinical trials. This can take some time. Once funding was secured, a process is developed, Good Manufacturing Practice (GMP) was used to produce high quality material, more formal animal experiments and toxicology studies are performed and then an Investigational New Drug (IND) application is filed. This process might take another 2-4 years. Then the venture can be started into Phase 1 trials (takes approximately two years), Phase 2 trials (takes approximately two years) and if everything looks great and the developer is sure the risk of failure is low, they embark on Phase 3 clinical trials (which takes about two years and is very expensive). Now, if Phase 3 data looks promising, a Biologics Licence Application (BLA) is filed with the United States Food and Drug Adminstration (USFDA) or National regulatory bodies to bring the vaccine to the market. For example, the National regulatory body in India is the Central Drugs Standard Control Organisation (CDSCO), led by the Drugs Controller General of India (DCGI), Government of India. Moreover, for International distribution of vaccines, a stamp of quality is required from the WHO, through its prequalification process. This might chew up another 1 or 2 years if licencing bodies request for additional data. They end up with about 15 years of development. Only then vaccine production can be started [7].
Given the seriousness of the pandemic, speedy manufacturing of the SARS-CoV-2 vaccine is the primary target. SARS-CoV-1 and MERS-CoV vaccine development data saved time and effectively skipped the initial stage of exploratory vaccine design. China made the first genomic sequence of the virus public on 11 January 2020 [8]. It was already known that the spike protein was the target. So, the fiddling around for years was skipped. This antigen was then just plugged into existing technology. In some cases, preclinical/toxicology data from similar vaccines were used for the initial research and development. Clinical phases were staggered, which speeds things a lot. Once a vaccine passes through Phase 3 clinical trial, it can be licenced the regular way or via an ‘Emergency Use Authorisation’ (EUA). The EUA allows the vaccine to be used before it is fully licenced based on available data that suggests a risk-benefit. It does not mean the vaccine is approved. It gives authorisation to use an unapproved vaccine during a state of emergency. EUA is an option for the SARS-CoV-2 vaccine. So, considering all this, several vaccines have been developed within just 1.5 years. Currently, two vaccines got approval via EUA for use in the United States.
Vaccine Testing and Approval Process
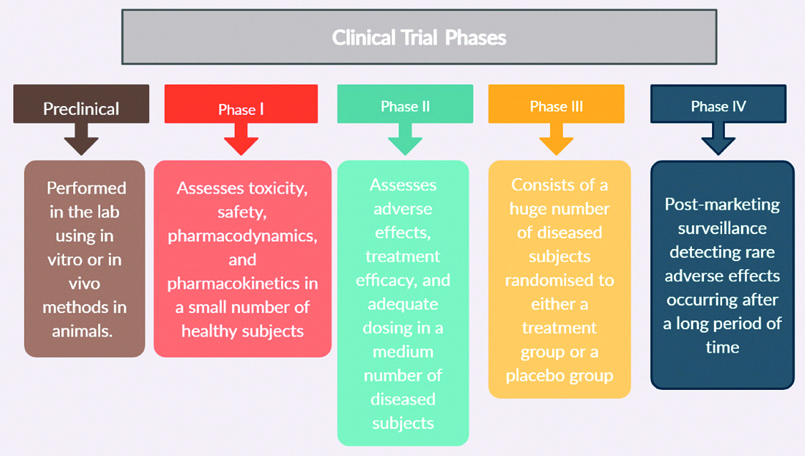
Clinical trial has four different phases [Table/Fig-2]. If postmarketing surveillance detects rare adverse effects, the vaccine needs to be withdrawn from circulation [9,10].
Process of clinical trial.
As of December 2020, WHO indicates that 61 vaccine candidates are currently undergoing clinical trials and 172 vaccine candidates are undergoing preclinical development. Out of 61, eleven are undergoing Phase 3 clinical trials [Table/Fig-3] [11-21], five are undergoing combined Phase 2/3, three are undergoing Phase 2, twenty are undergoing Phase 1/2 [Table/Fig-4] [22], and twenty-two are undergoing Phase 1 clinical trial. So, the globe is currently testing a whopping total of 233 different types of vaccines [23].
A few vaccines currently in Phase 3 clinical trials [11-21].
| Name | Company name | Type | Route | Results of trial |
|---|
| BNT162 | Pfizer, BioNTech | mRNA-based vaccine | I.M | Mulligan MJ et al., showed high antibody titre after vaccination in Phase 1/2 of the clinical trial [12].On 18 November, Pfizer announced interim results by a press release showing an efficacy of 95% (p<0.0001) in participants without prior SARS-CoV-2 infection [13].EUA was issued on 11 December for patients 16 years and older [14] |
| mRNA-1273 | Moderna | mRNA-based vaccine | I.M | Jackson LA et al., documented neutralising antibodies with mRNA-1273 in Phase 1 clinical trials. No trial-limiting adverse reaction was reported [15].On 16 November, Moderna announced interim results by a press release showing an efficacy rate of 94.5% (p <0.0001) among participants of Phase 3 COVE trial [16].Authorised for use in people >18 years under an EUA on 18 December [17] |
| AZD1222 | AstraZeneca; The University of Oxford; Serum Institute of India; IQVIA | Replication-deficient viral vector vaccine (adenovirus from chimpanzees) | I.M | Folegatti PM et al., showed that a single dose of vaccine produced anti-spike antibodies in 28 days. Mild and self-limiting side-effects were seen in Phase 1/2 clinical trials [18].On 23 November, AstraZeneca announced interim results by a press release demonstrating an efficacy rate of 70.4% in its Phase 3 trial [19]. |
| Ad5-nCoV | CanSino Biologics | Recombinant vaccine (adenovirus type 5 vector) | I.M | Zhu FC et al., found Ad5-nCoV to be safe and able to produce antibodies as well as a T-lymphocytic response after the first dose during Phase 1 clinical trials [20]. |
| CoronaVac | Sinovac | Inactivated vaccine (formalin with alum adjuvant) | I.M | Zhang YJ et al., reported the results of Phase 1/2 clinical trials. CoronaVac was immunogenic as well as safe [11] |
| No name announced | China National Pharmaceutical Group (Sinopharm); Wuhan Institute of Biological Products | Inactivated vaccine | I.M | Xia S et al., demonstrated vaccine-induced seroconversion during Phase 2 clinical trials. Transient injection site pain was reported [21]. |
A vaccine currently undergoing combined Phase 2/3 clinical trial [22].
| Name | Company name | Type | Route | Early results of trial |
|---|
| Bacillus Calmette-Guerin (BCG) vaccine | Radboud University Medical Center; University of Melbourne and Murdoch Children’s Research Institute; Faustman Lab at Massachusetts General Hospital | Live-attenuated vaccine | S.C | It’s a “repurposed vaccine” meaning the vaccine was already in use for tuberculosis but may also offer protection against COVID-19. Berg MK et al., showed that nations mandating BCG vaccination had a significantly slower spread of COVID-19 compared to nations that didn’t mandate BCG vaccination [22]. |
Various Vaccine Platforms
The main target of all vaccine developers is a vaccine that induces S-protein neutralising antibodies in the recipients. These antibodies will prevent the interaction of S protein with ACE 2 receptors. The vaccine candidates can be classified into traditional platforms (which are used for many licenced vaccines), modern platforms (which are used for some newer licenced vaccines), and novel platforms (which have never been used for a licenced vaccine).
1) Traditional Vaccine Platforms
Include live-attenuated vaccines and killed vaccines. Many licenced vaccines are designed on a traditional platform, which can be used to produce large quantities of coronavirus vaccine.
Killed/Inactivated Vaccine
SARS-CoV-2 is grown in a cell line (e.g., vero cells), harvested, and concentrated (using ultracentrifugation). It is then physically or chemically killed using formaldehyde or beta-propiolactone. It is a killed virus that still displays S protein on its surface.
Advantage: Unlike a live-attenuated virus, the killed virus has no risk of acquiring its virulence back. So, it is safer even if injected in an immunocompromised host.
Disadvantage: Since the virus is not alive, a weak immune response is produced compared to the live-attenuated vaccine. So, booster doses are needed. Also, virus production requires cell lines and (BSL-3) facilities. These infrastructures might be lacking in many developing nations.
Live-Attenuated Vaccine
SARS-CoV-2 virus is alive but weakened. Its virulence is decreased by culturing the SARS-CoV-2 virus in an unsuitable environmental condition. Virus is repeatedly passed through unfavourable conditions until it liked these conditions better than humans. Once injected in humans, it replicates to a limited extent but still induce a powerful immune response similar to one induced by natural infection. Instead of making viruses accustomed to nonhuman conditions, a rapid technology called “codon deoptimisation” can be used, in which the virulent gene of SARS-CoV-2 is deleted. So, it cannot cause infection but is still immunogenic.
Advantage: A cellular immune response that lasts longer is generated. If given into the nose, it can also produce secretory IgA which protects the gateway of virus entry.
Disadvantage: In immunocompromised, the virus can acquire back its virulence and can go rogue.
2) Modern Vaccine Platforms
Include protein-subunit vaccines and Virus-Like Particle (VLP) vaccines.
Protein Subunit Vaccine
The gene of the viral S antigen is isolated, injected into a suitable system (e.g., bacteria, mammalian cells, insect cells, yeast, or plants), and made to express itself. Translation of that sequence gives purified S protein of SARS-CoV-2. An example is NVX-CoV2373 (by Emergent BioSolutions). Receptor-Binding Protein (RBD) can also be made, which is a component of the spike protein.
Advantage: Does not involve contact with a live virus; so safer and has fewer side-effects.
Disadvantage: Cold-chain is required for the transportation of protein-subunit vaccines, maintained at 2-6°C, which increases overall cost of vaccine delivery. The vaccine loses its efficacy if it is exposed to room temperatures.
However, the Indian Institute of Science (IISc) has developed a protein vaccine that is thermo-tolerant. It’s a “warm vaccine”, meaning it stable at room temperature. They have developed an RBD protein vaccine that can be stored for months at room temperature (37°C). The thermo-stable RBD vaccine is currently undergoing preclinical development [24].
Virus-Like Particle (VLP) Vaccine
VLPs are molecules that have all external structural proteins of SARS-CoV-2 but lack the genome of SARS-CoV-2. So they are not infectious. But those S proteins on the surface trigger an immune response. In the lab, it’s like creating a hollow SARS-CoV-2 virus that is devoid of genetic material. Structural proteins of different viruses can be used to make a recombinant VLP. But the S protein should be one of the many proteins present on the surface of the hollow virus.
Advantage: Vaccine-like particles can’t replicate so VLPs are safer in comparison to live-attenuated vaccines.
Disadvantage: We need to produce different types of structural protein simultaneously to create a VLPs.
3) Novel Vaccine
Types include viral vector vaccines and nucleic acid-based vaccines.
Nonreplicating Viral Vector Vaccine
In this type of vaccine, the virus does not replicate in human cells nor infect humans, but just expresses the target antigens. Expression of antigen is done by introducing the genetic code for S protein in that vector’s genome. Later, that vector is introduced in the host. This process of mixing genes is called recombination. A common vector is nonreplicating adenovirus type-5. An example is Ad5-nCoV, used by CanSino Biologics.
Advantage: Just a single shot is required to produce a powerful immune response (humoral and cellular response). Animal virus vectors can be produced in large quantities since they grow easily in egg cells.
Disadvantage: If the patient had a past infection by the vector virus, then antibodies might interfere with vaccination. It has the highest incidence of side-effects when compared to other types of vaccines.
Replicating Viral Vector Vaccine
In this type of vaccine, the virus is capable of replicating in human cells. Its disease-causing genes are removed to make it harmless. The genes of the target protein are introduced. Once introduced in the host cell, it will express S protein. But it will also produce new viral particles, which will spread to infect new host cells that will also start expressing S protein. An example is TNX-1800, used by Toxin Pharmaceuticals.
Advantage: Replicating virus mimics a natural infection and provides a complete immune response (cellular, humoral, and mucosal immune response).
Disadvantage: The replicating viral vector itself can induce an immune response in the body.
Nucleic Acid-based Vaccines
It include DNA and RNA vaccines. Nucleic acid-based vaccine production just requires knowing the exact genetic sequence of the target protein. There is no need to cultivate the virus on cell lines and no BSL-3 facilities are needed. Thus, even without much infrastructure, it is possible to produce large amounts of doses by knowing the exact gene.
DNA Vaccine
Plasmid carrying the DNA encoding S protein is injected. Electric shock is applied (electroporation) to get that DNA into the cells of the vaccinee. Once incorporated into the host cell, DNA gets transcribed into mRNA. The mRNA is translated causing the host cell to express S protein. An example is INO-4800, used by Inovio Pharmaceuticals.
Advantage: The synthetic DNA is stable at room temperature and eliminates the requirement of a cold chain. No live virus handling is required. The DNA sequence can be changed in case the coronavirus undergoes an antigenic variation.
Disadvantage: Even though it elicits both humoral and cellular response, the antibody titre is low and multiple boosters are required. Vaccine production is limited by the speed of production of specific delivery devices (gene gun) needed to introduce the DNA.
RNA Vaccine
SARS-CoV-2 has a single-stranded, positive-sense RNA genome and therefore, the RNA molecule can be directly translated into protein. The mRNA encoding for S protein is injected into the host cell. It will get translated and the host cell will start expressing S protein. Immune cells will pick that S protein up and elicit a response. An example is mRNA-1273, used by Moderna.
Advantage: No live virus handling is required. Produces the highest antibody response, compared to all other vaccine types.
Disadvantage: More adverse reactions. The mRNA is unstable and gets degraded by endonucleases. Production is limited by carrier molecule supply (lipid nanoparticles). Strict cold chains are required to maintain the quality of the vaccine, thereby dramatically increasing delivery costs [5-7].
Potential Problems with Vaccines
Mucosal IgA and systemic IgG are generated via natural COVID-19 infection. IgG is specifically responsible for protecting the lungs, while IgA protects the mucosal surfaces of the upper respiratory tract. Most of the currently developed coronavirus vaccines are given via the intramuscular route. This route generates IgG but not IgA. So, most vaccines will protect against severe lung disease but still, upper respiratory tract infections can occur and spread it to others [7].
Older people do not respond well to vaccines, as documented by Sinovac’s Coronavac trial [11]. The geometric mean titres of antibodies were 60% lower in older individuals (65-85 years) in comparison to young individuals [25]. This hints at higher doses and multiple doses being required for older people.
Children show more side-effects compared to adults. This hints to lower doses being required for children [7].
SARS-CoV-2 is a novel virus, thus the population will require more than one dose to elicit an immune response. A prime-boost immunisation strategy will be needed with a prime dose, followed by a booster dose 3-4 weeks later. This leads to the issue of a large number of doses being required. The population of the world is almost 8 billion, so 16 billion doses will be required to be manufactured for delivering two shots per head. A single company will not be able to meet this demand. Another limiting step can be a shortage of glass vials, syringes and rubber stoppers [7].
Antibody-Dependent Enhancement (ADE)
It is a phenomenon in which antibodies created during a first-time infection, end up enhancing the disease rather than protecting against subsequent infections. This was seen with the dengue virus. ADE can be used by coronavirus as a means to cause more severe infection. Animal models have shown excess lung and liver damage after vaccination against SARS-CoV-2 [26].
In a vaccinated individual, the virus-antibody complex can bind to the Fc receptors, activate the complement system, or induce a conformational change in the glycoprotein of the viral envelope. This process triggers enhanced virus uptake into host cells (leading to increased viral infection and replication) due to the intensified binding efficiency of the virus-antibody [5,27]. This is why postmarketing surveillance is required to keep an eye on this phenomenon.
Recent surveys highlighted that uptake will not be universal even if an effective vaccine is made. In high-income nations, there is substantial vaccine skepticism. To gain herd immunity, this presents a significant challenge.
Herd Immunity
When a large number of immune individuals are present in a population, it indirectly protects the susceptible people. To achieve herd immunity against SARS-CoV-2, at least 67% of the population needs to be vaccinated [28]. So, even if the rest 30% of the population is unvaccinated, the others will stop the transmission of SARS-CoV-2 to them. So, it is like having a shield of vaccinated people who break the chain of transmission. Herd immunity does not require the whole population to be vaccinated [29].
Vaccine Hesitancy
It is defined as a delay in acceptance or refusal of vaccines despite the availability of vaccination services. Parents might refuse to have their children vaccinated against communicable diseases, even though it is established that a vaccine is safe and effective. Vaccine hesitancy presents risks to both the person and their community, as exposure to SARS-CoV-2 puts the individual at risk and if they are not vaccinated, they are far more likely to transmit the coronavirus to others. These are people with concerns about safety testing. People are hesitant to get a newly developed vaccine because they feel that it would still be too early to learn what long-term consequences it might have. They have a fear that vaccination causes autism, even though the researchers have proved the opposite [30]. Many people think that the rapid development of a vaccine is due to political pressure and does not have a scientific basis.
Anti-Vaxxers
It is a movement of people who challenge the protection of vaccines and legislation requiring compulsory vaccination. Under the disguise of defending personal freedom, they deceptively criticise public health initiatives [31]. In comparison to pro-vaccine pages, antivaccination Facebook pages are more in number and they generate antivaccine views among their followers [32].
The Vaccine Confidence Project is committed to “conducting a global study to track public opinion and emotions regarding COVID-19 vaccination” [33]. Low-income countries largely support vaccination. In South Asia and East Africa, about 90% of participants identified vaccination as safe and effective. This was in fact due to their citizen’s trust in their researchers, doctors, and nurses. This is in contrast to Western Europeans, where just 59% of respondents agree that vaccines are safe. 95% of respondents from China, Guatemala, Bangladesh, India, and Ethiopia agreed that childhood vaccines are important for the health of their children, according to research using the WHO SAGE Vaccine Hesitancy Scale. Western nations are on the opposite spectrum. Only half of the Americans are willing to get a COVID-19 vaccine [34].
To create trust and avoid vaccine hesitancy, governments, researchers, and global agencies should interact effectively with people. In resource-rich and resource-poor countries, vaccine efficacy is often variable. So, it is important that the vaccine developed to be used in poor countries shows effectiveness as well as safety. Also, if a country has shown a poor response against COVID-19, its people will have great mistrust in the vaccine provided by their government [35].
Missing Group from Trials
A study has shown that older adults are excluded from the majority of the COVID-19 vaccine trials. The ability to determine the effectiveness, dosage, and side effects of the COVID-19 vaccines would be impaired by such exclusion. Older adults are vulnerable to COVID-19 and many nursing homes consist almost entirely of elderly adults. Older adults will not trust a vaccine that’s not tested in their age group and this will increase vaccine hesitancy. This shows the importance of including older adults in the vaccine trial [36].
The creation of a vaccine for COVID-19 should not be the indicator of a successful response, nor should it signify the accomplishment of an improved public health system. The best indicator will be the ability to develop trust in the vaccine [37].
Vaccines in the Pipeline
The New York Times has developed a vaccine tracker that lists all the vaccines currently in the pipelines across the world [38]. Russia approved its home-grown vaccine Sputnik-V (Gam-COVID-Vac) for public use. Since, it was approved without undergoing a Phase 3 clinical trial, the safety of the vaccine is still questionable [39].
Once a vaccine hits the market, initially there would be a shortage of doses in comparison to the population. So, a physician should practice rationing while distributing the vaccine.
Normally, mass production of vaccines occurs only after the completion of the clinical trials. The urgent requirement has also led to rush this stage. Some pharmaceutical firms, though still undergoing clinical trials, are preparing to produce vast quantities of their vaccine candidates. This way, if their candidate successfully completes Phase 3, they can start distributing the already produced doses among people.
Experts have suggested that a COVID-19 vaccine will be available in early-to-mid- 2021, exactly 12 to 18 months after SARS-CoV-2 infection was first reported [40].
Western Nations Perspective on Distribution of Vaccines
A preliminary proposal for the strategic allocation of the coronavirus vaccine was issued by the Advisory Committee on Immunisation Practices (ACIP), CDC and WHO [41-43].
First preference must be given to medical personnel, pharmacists, emergency responders, firefighters, police, and army troops. Medical personnel are the ones who come in direct contact with COVID-19 patients.
Second preference must be given to morgue workers, communication and information technology sector employees, high-risk adults, and those related to making or distributing food (grocery store owners, meatpackers).
Individuals at increased risk of COVID-19 are older adults (aged 65 years or older) and adults with underlying medical conditions (aged 18 years or older). Underlying medical conditions are defined as obesity, hypertension, diabetes, kidney disease, cardiovascular disease, chronic lung disease, neurologic disease, immune suppression, asthma, autoimmune disease, and gastrointestinal/liver disease [41].
The third preference should be given to those living in assisted living facilities, long-term care facilities, and nursing homes. Kirkland, Washington has already reported an outbreak of COVID-19 in one of its nursing homes [44].
Fourth preference should be given to pregnant women, Hispanics, and African-Americans because they are likely to become seriously ill with COVID-19 and to die of it [45,46]. Minorities are affected more because they live in overcrowded homes, and have no access to clean water and healthcare services. So, early detection of COVID-19 and management is difficult. They do jobs that do not provide the chance to work from home and get paid sick-leave.
Children are in the low-risk group because young children seem to be less likely to spread the virus and have a high recovery rate [47]. In young children, ACE2 is immature and thus does not function properly as a receptor for SARS-CoV-2. Neonates and young children are asymptomatic [48]. So, they should be in a low priority group.
WHO has set resource allocation and priority-setting guidelines. They agree with the first preference being given to the healthcare workers and first responders because they help to restore the health of others.
What Makes Developing Nations Different?
Slums are home to one-third of the world’s population. Mumbai has about 20.5 million people, making it one of the most populated cities in the world. Out of it, 62% are slum dwellers [49]. The city authority of Mumbai found coronavirus antibodies in 57% of slum residents compared to 16% of nonslum residents of the same area [50]. Poor sanitation and lack of access to clean water help spread infectious diseases in slums, thus contributing to higher morbidity and mortality. Despite India being the vaccine manufacturing hub of the world, its immunisation coverage is still lower compared to other nations. For all developing nations, including India, the situation is dire, as almost 33% of the urban people live in unorganised ghettos. Owing to their greater burden of illness, denser living environments, and lack of access to health services, developing nations need to carve their plan for vaccine allocation. Priority groups will differ in developing nations. Overcrowding increases coronavirus transmission. The poor population has a low education level and they delay seeking treatment leading to severe illnesses [51]. Also, the health system gets over-burdened quickly in resource-limited areas so people find it difficult to get a bed. So, the only available action to break continued transmission is a vaccine.
Racial priority won’t stand true for developing nations. Instead, that group gets replaced by those living below the poverty line and in crowded places. The main obstacles to vaccination in low-income countries generally include the failure to access vaccines and equipment, as well as insufficient healthcare services and vaccination infrastructure. Moreover, these challenges are compounded during a pandemic by a global shortage of vaccines, the high price of the vaccines, and worsening inequity and inequality.
In urban slums, vaccination services tailored for the general public might not be as successful. Spreading awareness regarding vaccines play an important role. Increased internet access where often false information is spread leads to myths about vaccination. We should use social media platforms like Facebook, WhatsApp to clarify these misconceptions and share knowledge about vaccines. Religious leaders, local politicians, and auxiliary nurse midwives should provide vaccine education and myth-busters. These people belong to the same community and they speak the same language as locals so information becomes easier to digest. They use layman terminologies and descriptions which are received better by locals. Doctors and teachers should organise weekly meetings to spread awareness. Government should hire trained community health workers to give education, keeping in mind the cultural belief of the local people [52]. Since the slum population is largely affected, mass vaccinating them would break the chain of transmission of corona in the country. Vaccination centres should be made in close proximity to those epicentre areas. It will reduce the physical gap between the recipient and the physician. A mobile health clinic should be used to increase vaccination coverage in hard-to-reach areas. Financial incentives to encourage the uptake of vaccine should be provided. Low purchasing power prohibits slum dwellers from entering private hospitals. So, the government should subsidise the vaccine. Each nation should take measures to continually establish an understanding of vaccine hesitancy at the local level.
Who’s Role
COVAX is an initiative led by WHO, GAVI (formerly the Global Alliance for Vaccines and Immunisation), and CEPI (Coalition for Epidemic Preparedness Innovations). COVAX is a global access facility for vaccines. Once a vaccine is available, COVAX will mediate equitable access and distribution of these vaccines across all nations, irrespective of their purchasing power. It also helps nations build their manufacturing capacity and helps them to buy stock of vaccines even before it’s available in the market. It targets to distribute two million doses equally among all nations. Even low-income countries will have access to vaccines if they participate in the COVAX initiative. The first slot of the vaccine will be given to frontline workers. It will also keep a stock of 5% of the total available doses to tackle sudden outbreaks occurring across the world. This buffer stock will help to vaccinate refugees too. The good thing about COVAX is that rich countries will not be provided doses to vaccinate more than 20% of their population until all the participating nations have been offered doses to vaccinate 20% of their population. COVAX will end the acute phase of the COVID-19 pandemic, thus rebuilding the economies of all nations. Some nations will also be given an additional volume of doses if they have a higher COVID-19 burden and mortality [53,54].
Conclusion(s)
It is reassuring to know that many vaccine makers have good vaccine candidates based on varied platforms. They are being developed in different countries so not a single vaccine maker will be burdened to fulfill the demand of the world. First preference will be given to immunise healthcare workers and emergency responders. COVID-19 pandemic prompted researchers to use out-of-box methods to accelerate the vaccine development process and EUA will come in the picture once a vaccine candidate proves its worth. The development and distribution of vaccines needs international collaboration and monetary investments. This pandemic teaches us to prevent future zoonotic transmission of viruses into humans and to rapidly respond to newly emerged biohazards. Properly designed emergency plans are needed to produce and distribute a new vaccine within months, not years. If we win this race against the SARS-CoV-2 virus, our ability to react quickly to emerging viruses will be dramatically improved.
We should look for doubts, distrust, and misinformation about vaccines among people. Sharing scientific valid facts via communication should be done to tackle the problem of vaccine hesitancy. Only building confidence in new life-vaccine will improve its acceptance.
Developing nations should consider their own population’s requirements for prioritising vaccine allocation. Although we highlighted the issues with developing nations regarding vaccine distribution, each nation should consider the problems its people face while procuring a vaccine shot. Country-specific guidelines should take into account the purchasing power of its citizens, the hard-hit group as well as ethical principles. In urban slums, rapid growth and high population density call for a rising focus on vaccination for the urban people, where coronavirus spreads more rapidly.
[1]. Sifuentes-Rodríguez E, Palacios-Reyes D, COVID-19: The outbreak caused by a new coronavirusBol Med Hosp Infant Mex 2020 77(2):47-53.English10.24875/BMHIM.20000039 [Google Scholar] [CrossRef]
[2]. Who.int. [cited 2020 Dec 24]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 [Google Scholar]
[3]. Newman L, Maurice HillemanBMJ 2005 330(7498):102810.1136/bmj.330.7498.1028PMC557162 [Google Scholar] [CrossRef] [PubMed]
[4]. Fliesler N, How fast can we get a COVID-19 vaccine?Boston Children’s Discoveries [Internet]. Childrenshospital.org 2020 [cited 2020 Dec 24] Available from: https://discoveries.childrenshospital.org/covid-19-vaccine/ [Google Scholar]
[5]. Kaur SP, Gupta V, COVID-19 Vaccine: A comprehensive status reportVirus Res 2020 288:198114Epub 2020 Aug 1310.1016/j.virusres.2020.19811432800805 [Google Scholar] [CrossRef] [PubMed]
[6]. van Riel D, de Wit E, Next-generation vaccine platforms for COVID-19Nat Mater 2020 19(8):810-12.10.1038/s41563-020-0746-032704139 [Google Scholar] [CrossRef] [PubMed]
[7]. Krammer F, SARS-CoV-2 vaccines in developmentNature 2020 Sep 23 Epub ahead of print10.1038/s41586-020-2798-332967006 [Google Scholar] [CrossRef] [PubMed]
[8]. Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, Identification of a novel coronavirus causing severe pneumonia in human: A descriptive studyChin Med J (Engl) 2020 133(9):1015-24.10.1097/CM9.000000000000072232004165 [Google Scholar] [CrossRef] [PubMed]
[9]. Institute of Medicine (US) Committee on the Children’s Vaccine Initiative: Planning Alternative Strategies, Mitchell VS, Philipose NM, Sanford JPStages of vaccine development 1993 Washington, D.C., DCNational Academies Press [Google Scholar]
[10]. Who.int. [cited 2020 Dec 24]. Available from: https://www.who.int/biologicals/publications/clinical_guidelines_ecbs_2001.pdf?ua=1 [Google Scholar]
[11]. Zhang YJ, Zeng G, Pan HX, Li CG, Kan B, Hu YL, Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: Report of the randomized, double-blind, and placebo-controlled phase 2 clinical trialmedRxiv 2020 10.1101/2020.07.31.20161216 [Google Scholar] [CrossRef]
[12]. Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adultsNature 2020 586:589-93.Aug 12. Epub ahead of print10.1038/s41586-020-2639-432785213 [Google Scholar] [CrossRef] [PubMed]
[13]. Pfizer and BioNTech conclude phase 3 study of COVID-19 vaccine candidate, meeting all primary efficacy endpoints [Internet]Pfizer.com. [cited 2020 Dec 24]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine [Google Scholar]
[14]. Harkins: DM. Pfizer Inc. Attention: Ms. Elis a Harkins 500 Arcola Road [Internet]Fda.gov. [cited 2020 Dec 24]. Available from: https://www.fda.gov/media/144412/download [Google Scholar]
[15]. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, mRNA-1273 study group. An mRNA vaccine against SARS-CoV-2 - preliminary reportN Engl J Med 2020 383:1920-31.doi: 10.1056/NEJMoa2022483. Epub ahead of print. PMID: 32663912; PMCID: PMC7377258 [Google Scholar]
[16]. Moderna’s COVID-19 vaccine candidate meets its primary efficacy endpoint in the first interim analysis of the Phase 3 COVE study [Internet]. Modernatx.com. [cited 2020 Dec 24]. Available from: https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy [Google Scholar]
[17]. Fda.gov. [cited 2020 Dec 24]. Available from: https://www.fda.gov/media/144636/download [Google Scholar]
[18]. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Oxford COVID Vaccine Trial GroupSafety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trialLancet 2020 396(10249):467-78.doi: 10.1016/S0140-6736(20)31604-4. Epub 2020 Jul 20. Erratum in: Lancet. 2020 Aug 15;396(10249):466. PMID: 32702298; PMCID: PMC7445431 [Google Scholar]
[19]. AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19 [Internet]. Astrazeneca.com. 2020 [cited 2020 Dec 24]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html [Google Scholar]
[20]. Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trialLancet 2020 395(10240):1845-54.Epub 2020 May 2210.1016/S0140-6736(20)31208-3 [Google Scholar] [CrossRef]
[21]. Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trialsJAMA 2020 324(10):1-10.Epub ahead of print10.1001/jama.2020.1554332789505 [Google Scholar] [CrossRef] [PubMed]
[22]. Berg MK, Yu Q, Salvador CE, Melani I, Kitayama S, Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19Sci Adv 2020 6(32):eabc146310.1126/sciadv.abc146332923613 [Google Scholar] [CrossRef] [PubMed]
[23]. Draft landscape of COVID-19 candidate vaccines [Internet]. Who.int. [cited 2020 Dec 24]. Available from: https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines [Google Scholar]
[24]. Malladi SK, Singh R, Pandey S, Gayathri S, Kanjo K, Ahmed S, Design of a highly thermotolerant, immunogenic SARS-CoV-2 spike fragmentbioRxiv 2020 :08.15.25243710.1101/2020.08.15.252437 [Google Scholar] [CrossRef]
[25]. Walsh EE, Frenck R, Falsey AR, Kitchin N, Absalon J, Gurtman RNA-Based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy studymedRxiv [Preprint] 2020 2020:08.17.2017665110.1101/2020.08.17.20176651 [Google Scholar] [CrossRef]
[26]. Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, Immunisation with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virusPLoS One 2012 7(4):e35421doi: 10.1371/journal.pone.0035421. Epub 2012 Apr 20. Erratum in: PLoS One. 2012;7(8)10.1371/annotation/2965cfae-b77d-4014-8b7b-236e01a35492 [Google Scholar] [CrossRef]
[27]. Lee WS, Wheatley AK, Kent SJ, DeKosky BJ, Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapiesNat Microbiol 2020 5(10):1185-91.10.1038/s41564-020-00789-532908214 [Google Scholar] [CrossRef] [PubMed]
[28]. Randolph HE, Barreiro LB, Herd immunity: Understanding COVID-19Immunity 2020 52(5):737-41.10.1016/j.immuni.2020.04.01232433946 [Google Scholar] [CrossRef] [PubMed]
[29]. Episode #1 - Herd immunity [Internet]. Who.int. [cited 2020 Dec 24]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/media-resources/science-in-5/episode-1 [Google Scholar]
[30]. Hviid A, Hansen JV, Frisch M, Melbye M, Measles, Mumps, Rubella vaccination and autism: A nationwide cohort studyAnn Intern Med 2019 170(8):513-20.Epub 2019 Mar 510.7326/M18-210130831578 [Google Scholar] [CrossRef] [PubMed]
[31]. McAteer J, Yildirim I, Chahroudi A, The VACCINES act: deciphering vaccine hesitancy in the time of COVID-19Clin Infect Dis 2020 71(15):703-05.10.1093/cid/ciaa43332282038 [Google Scholar] [CrossRef] [PubMed]
[32]. Wadman M, Antivaccine forces gaining onlineScience 2020 368(6492):69910.1126/science.368.6492.69932409456 [Google Scholar] [CrossRef] [PubMed]
[33]. Vaccine Confidence Project [Internet]. Vaccineconfidence.org. [cited 2020 Dec 24]. Available from: https://www.vaccineconfidence.org/ [Google Scholar]
[34]. Cornwall W, Just 50% of Americans plan to get a COVID-19 vaccine. Here’s how to win over the restScience [Internet] 2020 [cited 2020 Dec 24] Available from: https://www.sciencemag.org/news/2020/06/just-50-americans-plan-get-covid-19-vaccine-here-s-how-win-over-rest10.1126/science.abd6018 [Google Scholar] [CrossRef]
[35]. Bhopal S, Nielsen M, Vaccine hesitancy in low- and middle-income countries: Potential implications for the COVID-19 responseArch Dis Child 2021 106(2):113-14.Epub ahead of print10.1136/archdischild-2020-31898832912868 [Google Scholar] [CrossRef] [PubMed]
[36]. Helfand BKI, Webb M, Gartaganis SL, Fuller L, Kwon CS, Inouye SK, The Exclusion of older persons from vaccine and treatment trials for coronavirus disease 2019-missing the targetJAMA Intern Med 2020 180(11):1546-49.Epub ahead of print10.1001/jamainternmed.2020.508432986099 [Google Scholar] [CrossRef] [PubMed]
[37]. Harrison EA, Wu JW, Vaccine confidence in the time of COVID-19Eur J Epidemiol 2020 35(4):325-30.Epub 2020 Apr 2210.1007/s10654-020-00634-332318915 [Google Scholar] [CrossRef] [PubMed]
[38]. Zimmer C, Corum J, Wee SL, Coronavirus vaccine trackerThe New York Times [Internet] 2020 Jun 10 [cited 2020 Dec 24] Available from: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html [Google Scholar]
[39]. Zimmer C, ‘this is all beyond stupid.’ experts worry about Russia’s rushed vaccineThe New York Times [Internet] 2020 Aug 11 [cited 2020 Dec 24] Available from: https://www.nytimes.com/2020/08/11/health/russia-covid-19-vaccine-safety.html [Google Scholar]
[40]. Gallagher J, Covid vaccine update: When will others be ready?BBC [Internet] 2020 Dec 8 [cited 2020 Dec 24] Available from: https://www.bbc.com/news/health-51665497 [Google Scholar]
[41]. Cdc.gov. [cited 2020 Dec 24]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-08/COVID-05-McLung.pdf [Google Scholar]
[42]. Bell BP, Romero JR, Lee GM, Scientific and ethical principles underlying recommendations from the advisory committee on immunisation practices for COVID-19 vaccination implementationJAMA 2020 324(20):2025-26.10.1001/jama.2020.2084733090194 [Google Scholar] [CrossRef] [PubMed]
[43]. WHO | Ethics and COVID-19: resource allocation and priority-setting. 2020 [cited 2020 Dec 24]; Available from: https://www.who.int/ethics/publications/ethics-and-covid-19-resource-allocation-and-priority-setting/en/ [Google Scholar]
[44]. McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, Public Health Seattle and King County, EvergreenHealth, and CDC COVID-19 investigation team. epidemiology of covid-19 in a long-term care facility in King County, WashingtonN Engl J Med 2020 382(21):2005-11.doi: 10.1056/NEJMoa2005412. Epub 2020 Mar 27. PMID: 32220208; PMCID: PMC7121761 [Google Scholar]
[45]. Millett GA, Jones AT, Benkeser D, Baral S, Mercer L, Beyrer C, Assessing differential impacts of COVID-19 on black communitiesAnn Epidemiol 2020 47:37-44.Epub 2020 May 1410.1016/j.annepidem.2020.05.00332419766 [Google Scholar] [CrossRef] [PubMed]
[46]. El Chaar M, King K, Galvez Lima A, Are black and Hispanic persons disproportionately affected by COVID-19 because of higher obesity rates?Surg Obes Relat Dis 2020 16(8):1096-99.10.1016/j.soard.2020.04.03832522406xs [Google Scholar] [CrossRef] [PubMed]
[47]. Rawat M, Chandrasekharan P, Hicar MD, Lakshminrusimha S, COVID-19 in newborns and infants-low risk of severe disease: Silver lining or dark cloud?Am J Perinatol 2020 37(8):845-49.Epub 2020 May 710.1055/s-0040-171051232380565 [Google Scholar] [CrossRef] [PubMed]
[48]. Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epitheliaJ Virol 2005 79(23):14614-21.10.1128/JVI.79.23.14614-14621.200516282461 [Google Scholar] [CrossRef] [PubMed]
[49]. Mumbai Population 2020 [Internet]. Worldpopulationreview.com. [cited 2020 Dec 24]. Available from: https://worldpopulationreview.com/world-cities/mumbai-population [Google Scholar]
[50]. Guardian staff reporter. Half of Mumbai’s slum residents have had coronavirus study. The guardian [Internet]. 2020 Jul 29 [cited 2020 Dec 24]; Available from: http://www.theguardian.com/world/2020/jul/29/half-of-mumbais-slum-residents-have-had-coronavirus-study [Google Scholar]
[51]. Singh S, Sahu D, Agrawal A, Vashi MD, Ensuring childhood vaccination among slums dwellers under the National Immunisation Program in India - Challenges and opportunitiesPrev Med 2018 112:54-60.Epub 2018 Apr 410.1016/j.ypmed.2018.04.00229626558 [Google Scholar] [CrossRef] [PubMed]
[52]. Gurnani V, Haldar P, Aggarwal MK, Das MK, Chauhan A, Murray J, Improving vaccination coverage in India: lessons from Intensified Mission Indradhanush, a cross-sectoral systems strengthening strategyBMJ 2018 363:k478210.1136/bmj.k478230530467 [Google Scholar] [CrossRef] [PubMed]
[53]. COVAX [Internet]. Who.int. [cited 2020 Dec 24]. Available from: https://www.who.int/initiatives/act-accelerator/covax [Google Scholar]
[54]. Fair allocation mechanism for COVID-19 vaccines through the COVAX Facility [Internet]. Who.int. [cited 2020 Dec 24]. Available from: https://www.who.int/publications/m/item/fair-allocation-mechanism-for-covid-19-vaccines-through-the-covax-facility [Google Scholar]