Introduction
With the advent of SARS CoV-2, much research has been done in formulating preventive and therapeutic strategies in the form of drugs inhibiting viral entry into host cells, viral Protease inhibitors, viral RdRp inhibitor, import in channel inhibitors, drugs with multicentric action, immuno-modulators, and adjuvants. Other management modalities like regenerative medicine and the use of convalescent plasma therapy have also come up.
Potential Drugs for Therapeutic and Preventive Management of COVID-19
1. Viral Entry Inhibitors (Targeting Transmembrane Serine Protease 2 (TMPRSS2) and Viral Spike Glycoprotein)
TMPRSS2 is highly expressed on cells of epithelial cells of lungs [1] and subsegmental bronchial branches [2] while Angiotensin-Converting Enzyme 2 (ACE2) is present on both Pneumocytes 1 and 2 [3] while also on transient secretory cells of subsegmental bronchial branches that have high Rho Guanosine Triphosphatase (GTPase) activity making them vulnerable to infection [2]. The spike protein of SARS CoV 2 undergoes proteolytic activation by numerous host proteases like Elastase, Cathepsins, and Trypsin, TMPRSS 2 [4,5] and together with the help of ACE2 infects the pneumocytes [3,6].
The following drugs target this interaction
• Nafamostat (Camostat Mesilate):
Mechanism of action: It is a synthetic Serine Protease Inhibitor developed for the treatment of chronic pancreatitis [7] and oral squamous cell carcinoma [8,9]. It can target the TMPRSS2 and can be used as a potential drug for treatment [10]. It also inhibits intravascular coagulopathy hence can prevent microvascular thrombosis in patients of severe COVID-19 [11].
Trials and outcomes: Outcomes from a case series show low mortality in patients of severe COVID-19 on combination therapy of Favipiravir and Nafamostat [12].
Adverse events: Case reports of Nafamostat induced hyperkalaemia have been reported [13].
Final verdict: The combination of Favipiravir and Nafamostat should be considered for further research for their use in decreasing mortality in severely ill patients of COVID-19. Nafamostat should be administered with close monitoring of serum potassium levels.
• Umifenovir (Arbidol)
Mechanism of action: This drug blocks the trimerization of Spike glycoprotein31 and prevents viral entry.
Trials and outcomes: A study showed that while on Arbidol monotherapy, patients showed no viral load after day 14, this is in contrast to patients on Lopinavir (LPV)/Ritonavir (44.1% of whom showed viral load) [14]. A retrospective cohort study showed favourable response with improvement in Chest CT of patients (69% in LPV/r and Arbidol combination group Vs 29% in Arbidol monotherapy group) receiving Arbidol with LPV/r, but another observational study later stated that the combination may not benefit significantly in clinical improvement of patients [15,16].
Adverse events: No severe side-effects were reported in an exploratory randomised and controlled study of 30 patients, where all of the patients recovered and were found to be healthy at follow-up [17].
Final verdict: Umefenovir has only mild to moderate adverse effects like diarrhoea and nausea (11% patients in Umifenovir group Vs 8% patients in control groups as seen in a retrospective study), and can be considered for further trials to determine its clinical efficacy in combination with LPV/r [18].
Experimental inhibitors: DX600 is a potent inhibitor that is human ACE2 specific [6,7]. Many other molecules like Small peptides and tripeptides, MLN-4760, N-(2-aminoethyl)-1 aziridine-ethanamine [8,9] and the TNF-α Converting Enzyme (TACE) have also been found to be potent human ACE2 inhibitors in-vitro [10,14,19,20]. Another candidate is phytochemical Nicotianamine (present in soybean, hence dietary in origin and completely safe) that also inhibits ACE2 [21,22]. The latest development is a recombinant human ACE2 protein (hrsACE2) that prevents infection of engineered human capillary and kidney organoids [23].
2. Aminoquinolines with Multicentric Action
Hydroxychloroquine (HCQ) and Chloroquine (CQ) are known to mankind since 1939 when it was substituted Quinacrine for treatment of Malaria during the end of World War 2 [22,24].
Mechanism of action: The suggested mechanism involves the anti-inflammatory, antiviral and inhibition of viral entry by HCQ [Table/Fig-1] [25-27].
Shows the suggested mechanism of action of HCQ in SARS CoV-2.
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; ACE2: Angiotensin-Converting Enzyme 2; HCQ: Hydroxychloroquine; BCR: Breakpoint Cluster Region; TCR: T-cell receptor
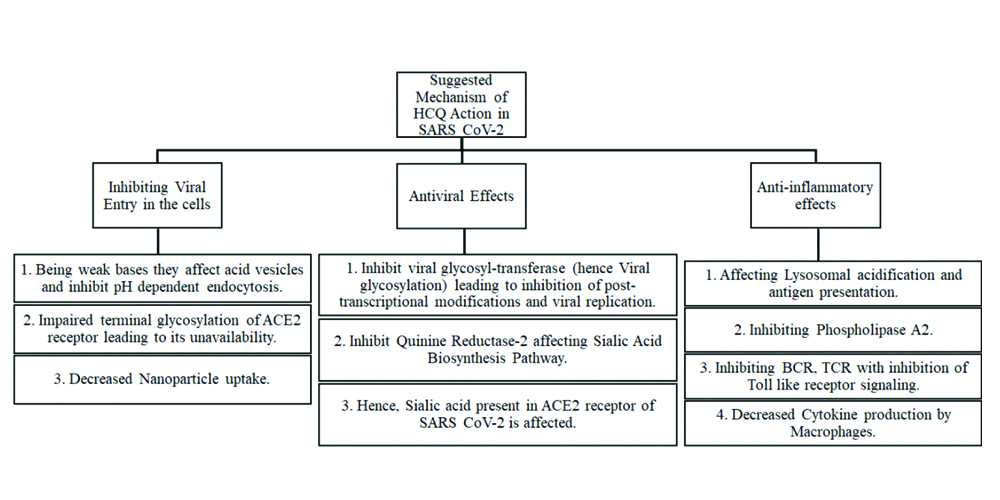
Trials and outcomes: Initial clinical trials (mostly conducted in the month of Feb-March 2020) showed increased viral clearance with HCQ usage [28], significant reduction in fever, and shortening of recovery time and cough remission time with a larger proportion of people with pneumonia showing improvement with better radiological features [29]. Also, it was seen that CRP declined faster with HCQ [30]. Newer studies (May-June 2020) suggest a different picture. While the combination of HCQ with Azithromycin which was initially claimed to guarantee 100% viral clearance [28] in six patients’ nasopharyngeal swabs, a new study on 11 patients on the same drug combination showed shocking responses with almost all patients remaining positive for SARS CoV2 as found on their nasopharyngeal swabs. In one patient, QT prolongation was seen, while another one patient succumbed to COVID-19 [31].
In another open-label randomised trial on 150 laboratory confirmed cases of COVID-19, comparing standard care with HCQ against standard care alone, a difference of 4.1% was seen in the negative conversion by 28 days, while in case of adverse effects, the difference was 21%. The study concluded that HCQ does not increase the significant probability of negative conversion in mild to moderate infection while causing deleterious adverse events [30].
In a randomised trial including 821 asymptomatic patients, 87.6% of whom had confirmed high-risk exposure to SARS CoV-2, the incidence of illness in the group which received HCQ prophylaxis was found to be 11.8% against 14.3% in the placebo group. Hence, there was not a convincing absolute difference of -2.4% [32]. But the problem is “off label use” which itself has reported 102 adverse cardiac events, 86% with HCQ alone or 60% in combination with Azithromycin [33]. But recently, CQ was found to be only effective in protecting virus-induced cytopathic effect at around 30 μM with a therapeutic index of 1.5 [34].
Adverse events: The common adverse effects associated with these drugs include gastrointestinal upset, more with HCQ than CQ [35,36]. Retinopathy is seen only on chronic use or high dose (>5mg/kg) [37], therefore, no ophthalmological screening is required for patients [38]. The most fatal side-effect is QTc prolongation and fatal Ventricular arrhythmias [39].
Final verdict: The viral clearance by CQ and its prophylactic use is doubtful and needs further evaluation. It should be administered with care due to its serious adverse effects and should be strictly avoided in cardiac patients.
3. Triple Combination of an Anti-Inflammatory/Anti-Neoplastic with Anti-Parasitic and Anti-Helminthic
The spike protein of Coronavirus GX_P2V shares 92.6% identity with SARS CoV-2 hence using it as a model. Cepharanthine demonstrated the most potent resistance to GX_P2V infection by inhibiting both viral entry and replication. It is also suggestive of effectivity against pan-betacoronavirus [40]. Examples include Cepharanthine (anti-inflammatory anti-neoplastic alkaloid) [41], Selamectin/Ivermectin (Antiparasitic and Anti-helminthic) [42], and Ivermectin.
Ivermectin is also seen to inhibit Importin channel required for entry of RNA dependent RdRp in the nucleus [42], inhibiting SARS CoV-2 in-vitro [43]. Mefloquine (used for the treatment of malaria) [44] showed complete inhibition of cytopathic effects in cell culture [40]. Another strong evidence comes from the effectiveness of Cepharanthine along with its homologous (natural alkaloids) anticancer agents like bis-benzylisoquinoline tetrandrine (TET) and fangchinoline (FAN) against human coronavirus strain OC43 strain in MRC-5 human lung cells [45]. The OC43 strain causes 15-30% of mild upper respiratory infections in humans [46].
4. Viral Protease Inhibitors
Mechanism of action: LPV is used with Ritonavir(r) as a booster. These are viral protease inhibitors, hence the combination LPV/r has been found to inhibit the virus in-vitro [47].
Trials and outcomes: In a study where LPV/r was administered to patients, it was observed that there was reduction in need of supplemental oxygen in 60% patients (3 out of 5) within 3 days. In the same study, it was also seen that the viral shedding in Nasopharyngeal swab was cleared in 40% patients within 2 days of treatment by LPV/r [46]. In another trial on 199 patients (99 on LPV/r and 100 on standard care), mortality with LPV/r was 19.2% and 25% in a control group (-5.8% percentage group). Patients with detectable viral RNA were similar in both groups at various points. Median time to clinical improvement was shortened by 1 day in LPV/r group [48].
Adverse events: Patients on LPV/r may have minor gastrointestinal adversities [48].
Final verdict: Triple combination of LPV/r, Interferon Beta-1b, and Ribavirin is superior in alleviating symptoms and shortening viral shedding [49].
5. RNA Polymerase Inhibitor
Favipiravir
Mechanism of action: It is a nucleotide analog which is a broad-spectrum inhibitor of RNA Dependent RNA Polymerase (RdRp) of RNA viruses. It has been proved effective against influenza, yellow fever virus, arena virus, bunyavirus, foot and mouth viruses [50]. In context to Ebola virus, its monotherapy proved ineffective in very high viremia while intermediate and high viremia need meritorious investigation. Based on a study, in Group A where Ct (cyclic threshold) was <20, mortality was 7% higher and no decrease in viral load was seen; while in a Group with Ct ≥20, mortality was 33% lower with rapid viral clearance [51]. Based on its known efficacy in RNA viruses; it became a prime candidate for investigation against SAR CoV-2.
Trials and outcomes: In a randomised study on 240 patients, there was not much difference in clinical recovery rate with Favipiravir (71/116) compared to Arbidol (62/120), while Favipiravir reduce the latencies of fever and cough [52].
Adverse events: The common adverse effects include increased serum uric acid levels [50]. This drug also affects QT interval [53].
Final verdict: Patients treated with Favipiravir have faster viral clearance and better chest imaging changes when compared to patients treated with LPV/r [51]. Favipiravir may be used in combination with other drugs to treat COVID-19, although more such detailed studies are needed.
Remdesivir
Mechanism of action: It is a nucleotide analog inhibiting RdRp, which was devised for the treatment of Ebola and Marburg virus infection. It has a broad-spectrum action against members of Filoviridae and Coronaviridae in nonclinical models. In-vitro, it is effective against SARS CoV-2 with EC50 of 1.76 uM in Vero E6 cells [54].
Trials and outcomes: Its first use for treating COVID-19 cases in the US showed significant effectiveness [55] and soon in a study of a cohort of patients of severe COVID-19 infection, compassionate use of Remdesivir showed clinical improvement in 68% patients [56]; while another study showed that it was associated with numerically faster clinical improvement [57]. In an evaluation of 19 antiviral drugs, Remdesivir was found to be the most effective drug [34].
Adverse events: In a cohort of 61 patients, a total of 32 patients reported adverse effects, the most common being increased hepatic enzymes, diarrhoea, rash, hypotension, and renal impairment [56].
Final verdict: Remedisvir can be the potential drug for clinical treatment of COVID-19 as it has shown appreciable clinical improvement in patients.
Other RdRp inhibitors like Ribavirin [49,58], Beta hydroxycytidine [59], and Merimepodib [60] need to undergo further trials.
6. Immunomodulators
An important example of HCQ as immunomodulators has already been discussed above [Table/Fig-1]. Emerging studies suggest that the damage associated with COVID-19 stems from the release of pro-inflammatory cytokines leading to damage to lung tissue [61-67].
Corticosteroids
Mechanism of action: These are having broad anti-inflammatory activity.
Trials and outcomes: A retrospective study was conducted on 401 patients, where 152 were critical and 249 were noncritical. One hundred twenty one patients out of 152 critical patients (79.6%) received steroids and 25 out of them (20.7%) died; 147 of 249 noncritical patients (59%) received steroids and there were no deaths in this group. This data suggested that there was no significant reduction in death rate or hospital stay in patients but when analysed with a focus on 152 critical patients, steroids lead to reduced overall mortality rates and shorter hospital stay [68].
Adverse events: The adverse effects of steroids are inevitable, a retrospective cohort study showed higher adverse events in the group treated with corticosteroids (37.9 Vs 16.7%). This study also showed a very high mortality (26.9%) and even increase plasma viral load in non-ICU patients [69].
Final verdict: All these data analyses suggest judicious use of steroids. Too early administration of steroids will suppress the body’s defense and lead to increase plasma viral load, resulting in severe adverse consequences; while use in critical patients with features of pro-inflammatory cytokine storm will lead to suppression of excessive inflammation. Hence, in patients with deteriorating oxygenation, imaging studies, and excessive inflammation, the use of glucocorticoids is considered appropriate [70].
Tocilizumab and Sarilumab are anti-Interleukin (IL)-6 and have a therapeutic effect on infection-induced cytokine storm. As IL-6 is detected in high levels in COVID-19 patients, hence these drugs seem to be promising [71].
Bevacizumab, an anti-Vascular Endothelial Growth Factor (VEGF), is under exploration. COVID-19 patients have elevated VEGF and it is believed it can play a key role in the pathogenesis of the disease [72].
7. Potential Miscellaneous Adjuvants
Plitidepsin inhibits Elongation factor [73]
Bemcentinib is an Anexelekto (AXL) Kinase inhibitor [74]
Rintatolimod is a Toll Like Receptor 3 (TLR)-3 Inhibitor [75]
Melatonin has been known for its anti-inflammatory, anti-oxidative and immune-modulative response with a high safety profile hence is a potential candidate for use as an adjuvant in COVID-19 patients [76]
Regenerative Medicine
Exosomes derived from allogeneic bone marrow mesenchymal stem cells for the treatment of COVID-19 have been tried and have shown promising results, with 71% of patients showing recovery/discharge after a mean 5.6 days post iv infusion of ExoFlo. There were no severe adverse effects. In this study, where bmMSC derived exosomes were used in an inpatient setting is the first known clinical study of its own kind. This branch of regenerative medicine shows hope for treatment of not only COVID-19 but a myriad of inflammatory diseases [77].
Convalescent Plasma Therapy (CPT)
CPT has long been used for management of viral infection like H1N1 [78], SARS [79], Ebola [80], H5N1 [81].
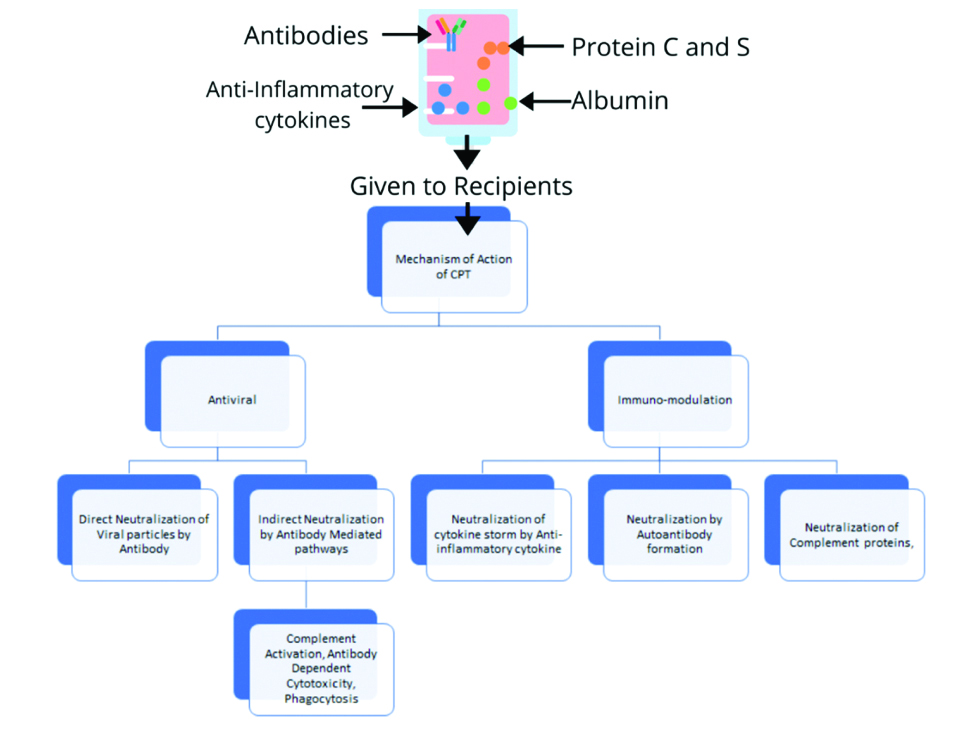
Mechanism of action: It is proposed that high antibodies from patients recovering from these infections might suppress viremia and salvage other patients [Table/Fig-2] [82].
Proposed mechanism of CPT in COVID-19 patients.
CPT: Convalescent plasma therapy
Trials and outcomes: A study shows that one dose of 200 mL CP is well tolerated and results in better oxygenation within three days with rapid neutralisation of viremia [83]. The key factor lies in neutralisation antibody titer and treatment time point. There are no severe adverse effects seen with CPT [83]. In a breakthrough study on five critically ill patients, it was seen that post-CPT fever solved in three days (4/5 patients), Arterial Oxygen Partial Pressure/Fractional Inspired Oxygen (Pao2/Fio2) increased with negative viral load and resolution of ARDS occurred within 12 days [84]. CPT can act as a last resort for salvaging critically ill patients. Another clinical randomised trial on 103 patients was conducted where 101 patients completed the trial. They were randomised in CPT and Control groups in a 1:1 ratio. 23 patients in CPT group and 22 patients in control group had severe COVID-19 while 29 patients in both CPT and control group had life-threatening COVID-19. The study showed clinical improvement in 53.9% in the CPT group (27/52) vs. 43.1% in the control group (22/51); while in severely affected individuals of each group, there was clinical improvement in 91.3% in the CPT group (21/23) vs. 68.2% in the control group (15/22). For patients suffering from life-threatening COVID-19, clinical improvement was seen in 20.7% (6/29) of the CPT group vs 24.1% (7/29) of control group. This which clearly shows that CPT therapy is very beneficial in severe patients of COVID-19 [85].
There is a direct effect of neutralising Antibody that binds to the COVID-19 Receptor binding domain without any competition with ACE-2 [86]. It is also seen that some antibodies inhibit the complement cascade and the formation of immune complexes, also neutralise IL-1β and TNF-α thereby suppressing the systemic inflammation [87-89]. Antibody-Dependent Enhancement (ADE) is a phenomenon where pre-existing neutralising antibodies favours viral replication in a host cell with help of Fragment Crystallizable (Fc) and Complement receptor interaction [90] and is seen in feline coronaviruses and is a concern to be investigated, however, no reports of ADE in Human coronaviruses is found.
Dosage of CPT
An optimal dose cannot be determined due to variability of factors involved in various case reports. It usually ranges from a single dose of 200 mL plasma with neutralising antibody titer>1:640 while another report stated a maximum of use of 2400 mL plasma in a 73-year-old patient [79,91,92].
Conclusion(s)
Remedisvir has shown very positive outcomes with minimal adverse events. Combination therapies of LPV/r or Favipiravir with Nafamostat are emerging as possible therapies for patients of COVID-19. The clinical efficacy of HCQ is highly questionable and needs further evaluation. HCQ, steroids need to be carefully administered to patients. CPT therapy is beneficial for salvaging severe patients of COVID-19 because of its antiviral and immuno-modulatory effects. Newer advancements like regenerative medicine is very promising but has a long way to go. There has been extensive research and development going on to develop the best therapeutic, prophylactic and preventive treatment modalities for SARS CoV-2.
[1]. Paoloni-Giacobino A, Chen H, Peitsch MC, Rossier C, Antonarakis SE, Cloning of the TMPRSS2 gene, which encodes a novel serine protease with transmembrane, LDLRA, and SRCR domains and maps to 21q22.3Genomics 1997 44(3):309-20.10.1006/geno.1997.48459325052 [Google Scholar] [CrossRef] [PubMed]
[2]. Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cellsEMBO J 2020 39(10):e10511410.15252/embj.20105114 [Google Scholar] [CrossRef]
[3]. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesisJ Pathol 2004 203(2):631-37.10.1002/path.157015141377 [Google Scholar] [CrossRef] [PubMed]
[4]. Li F, Structure, function, and evolution of coronavirus spike proteinsAnnu Rev Virol 2016 3(1):237-61.10.1146/annurev-virology-110615-04230127578435 [Google Scholar] [CrossRef] [PubMed]
[5]. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitorCell 2020 181(2):271-280.e8.10.1016/j.cell.2020.02.05232142651 [Google Scholar] [CrossRef] [PubMed]
[6]. Pedersen KB, Sriramula S, Chhabra KH, Xia H, Lazartigues E, Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assaysAm J Physiol-Regul Integr Comp Physiol 2011 301(5):R1293-99.10.1152/ajpregu.00339.201121880865 [Google Scholar] [CrossRef] [PubMed]
[7]. Huang L, Sexton DJ, Skogerson K, Devlin M, Smith R, Sanyal I, Novel peptide inhibitors of Angiotensin-converting Enzyme 2J Biol Chem 2003 278(18):15532-40.10.1074/jbc.M21293420012606557 [Google Scholar] [CrossRef] [PubMed]
[8]. Trask AJ, Groban L, Westwood BM, Varagic J, Ganten D, Gallagher PE, Inhibition of angiotensin-converting enzyme 2 exacerbates cardiac hypertrophy and fibrosis in Ren-2 hypertensive ratsAm J Hypertens 2010 23(6):687-93.10.1038/ajh.2010.5120300067 [Google Scholar] [CrossRef] [PubMed]
[9]. Minghao Y, Jan W, Francisco GP, Mahmoud S, Karla E, Laura GH, Murine recombinant angiotensin-converting enzyme 2Hypertension 2012 60(3):730-40.10.1161/HYPERTENSIONAHA.112.19862222777933 [Google Scholar] [CrossRef] [PubMed]
[10]. Guy JL, Jackson RM, Jensen HA, Hooper NM, Turner AJ, Identification of critical active-site residues in angiotensin-converting enzyme-2 (ACE2) by site-directed mutagenesisFEBS J 2005 272(14):3512-20.10.1111/j.1742-4658.2005.04756.x16008552 [Google Scholar] [CrossRef] [PubMed]
[11]. Turshudzhyan A, Anticoagulation options for Coronavirus Disease 2019 (COVID-19)-induced coagulopathyCureus 2020 12(5):e8150 [Google Scholar]
[12]. Doi K, Ikeda M, Hayase N, Moriya K, Morimura N, Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case seriesCrit Care [Internet] 2020[cited 2020 Dec 14] :24Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7332736/10.1186/s13054-020-03078-z32620147 [Google Scholar] [CrossRef] [PubMed]
[13]. Okajima M, Takahashi Y, Kaji T, Ogawa N, Mouri H, Nafamostat mesylate-induced hyperkalemia in critically ill patients with COVID-19: Four case reportsWorld J Clin Cases 2020 8(21):5320-25.10.12998/wjcc.v8.i21.532033269265 [Google Scholar] [CrossRef] [PubMed]
[14]. Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19J Infect 2020 81(1):e21-23.10.1016/j.jinf.2020.03.06032283143 [Google Scholar] [CrossRef] [PubMed]
[15]. Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort studyJ Infect [Internet] 2020 Mar 11 [cited 2020 Jun 15] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7156152/ [Google Scholar]
[16]. Wen CY, Xie ZW, Li YP, Deng XL, Chen XT, Cao Y, [Real-world efficacy and safety of lopinavir/ritonavir and arbidol in treating with COVID-19: an observational cohort study]Zhonghua Nei Ke Za Zhi 2020 59(0):E012 [Google Scholar]
[17]. Yethindra V, Tagaev T, Uulu MS, Parihar Y, Efficacy of umifenovir in the treatment of mild and moderate COVID-19 patientsInt J Res Pharm Sci 2020 11(SPL1):506-09.10.26452/ijrps.v11iSPL1.2839 [Google Scholar] [CrossRef]
[18]. Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q, Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective studyClin Microbiol Infect 2020 26(7):917-21.10.1016/j.cmi.2020.04.02632344167 [Google Scholar] [CrossRef] [PubMed]
[19]. Mores A, Matziari M, Beau F, Cuniasse P, Yiotakis A, Dive V, Development of potent and selective phosphinic peptide inhibitors of angiotensin-converting enzyme 2J Med Chem 2008 51(7):2216-26./10.1021/jm701275z18324760 [Google Scholar] [CrossRef] [PubMed]
[20]. Haga S, Nagata N, Okamura T, Yamamoto N, Sata T, Yamamoto N, TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compoundsAntiviral Res 2010 85(3):551-55.10.1016/j.antiviral.2009.12.00119995578 [Google Scholar] [CrossRef] [PubMed]
[21]. Takahashi S, Yoshiya T, Yoshizawa-Kumagaye K, Sugiyama T, Nicotianamine is a novel angiotensin-converting enzyme 2 inhibitor in soybeanBiomed Res 2015 36(3):219-24.10.2220/biomedres.36.21926106051 [Google Scholar] [CrossRef] [PubMed]
[22]. Cord N, McKee DL, The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol, and GenisteinCurrent Medicinal Chemistry 2020 27Bentham Science Publishers:1[cited 2020 Jun 23]. Available from: https://www.ingentaconnect.com/content/ben/cmc/pre-prints/content-3210799110.2174/092986732766620022811073832107991 [Google Scholar] [CrossRef] [PubMed]
[23]. Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2Cell 2020 181(4):905-13.e7.10.1016/j.cell.2020.04.00432333836 [Google Scholar] [CrossRef] [PubMed]
[24]. Tanenbaum L, Antimalarial agents: Chloroquine, Hydroxychloroquine, and QuinacrineArch Dermatol 1980 116(5):58710.1001/archderm.1980.016402900970266990871 [Google Scholar] [CrossRef] [PubMed]
[25]. Gies V, Bekaddour N, Dieudonné Y, Guffroy A, Frenger Q, Gros F, Beyond anti-viral effects of Chloroquine/HydroxychloroquineFront Immunol 2020 11:140910.3389/fimmu.2020.0140932714335 [Google Scholar] [CrossRef] [PubMed]
[26]. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)Clin Infect Dis Off Publ Infect Dis Soc Am 2020 71(15):732-39.10.1093/cid/ciaa23732150618 [Google Scholar] [CrossRef] [PubMed]
[27]. Quiros Roldan E, Biasiotto G, Magro P, Zanella I, The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): A role for iron homeostasis?Pharmacol Res 2020 158:10490410.1016/j.phrs.2020.10490432430286 [Google Scholar] [CrossRef] [PubMed]
[28]. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomised clinical trialInt J Antimicrob Agents 2020 2020:10594910.1016/j.ijantimicag.2020.10594932205204 [Google Scholar] [CrossRef] [PubMed]
[29]. Saleh M, Gabriels J, Chang D, Kim BS, Mansoor A, Mahmood E, Effect of Chloroquine, Hydroxychloroquine and Azithromycin on the corrected QT interval in patients with SARS-CoV-2 infectionCirc Arrhythm Electrophysiol 2020 13(6):e00866210.1161/CIRCEP.120.008662 [Google Scholar] [CrossRef]
[30]. Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trialBMJ 2020 369:m184910.1136/bmj.m184932409561 [Google Scholar] [CrossRef] [PubMed]
[31]. Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infectionMédecine Mal Infect 2020 50(4):38410.1016/j.medmal.2020.03.00632240719 [Google Scholar] [CrossRef] [PubMed]
[32]. Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, A randomised trial of Hydroxychloroquine as postexposure prophylaxis for Covid-19N Engl J Med 2020 383(6):517-25.10.1056/NEJMoa201663832492293 [Google Scholar] [CrossRef] [PubMed]
[33]. Gérard A, Romani S, Fresse A, Viard D, Parassol N, Granvuillemin A, “Off-label” use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: A survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance CentersTherapies [Internet] 2020 May 7 [cited 2020 Jun 23] Available from: http://www.sciencedirect.com/science/article/pii/S004059572030091310.1016/j.therap.2020.05.00232418730 [Google Scholar] [CrossRef] [PubMed]
[34]. Liu S, Lien CZ, Selvaraj P, Wang TT, Evaluation of 19 antiviral drugs against SARS-CoV-2 InfectionbioRxiv 2020 2020.04.29.067983 [Google Scholar]
[35]. Furst DE, Lindsley H, Baethge B, Botstein GR, Caldwell J, Dietz F, Dose-loading with hydroxychloroquine improves the rate of response in early, active rheumatoid arthritis: A randomised, double-blind six-week trial with eighteen-week extensionArthritis Rheum 1999 42(2):357-65.10.1002/1529-0131(199902)42:2<357::AID-ANR19>3.0.CO;2-J [Google Scholar] [CrossRef]
[36]. Arnaout A, Robertson SJ, Pond GR, Lee H, Jeong A, Ianni L, A randomised, double-blind, window of opportunity trial evaluating the effects of chloroquine in breast cancer patientsBreast Cancer Res Treat 2019 178(2):327-35.10.1007/s10549-019-05381-y31392517 [Google Scholar] [CrossRef] [PubMed]
[37]. Jorge A, Ung C, Young LH, Melles RB, Choi HK, Hydroxychloroquine retinopathy- implications of research advances for rheumatology careNat Rev Rheumatol 2018 14(12):693-703.10.1038/s41584-018-0111-830401979 [Google Scholar] [CrossRef] [PubMed]
[38]. Marmor MF, COVID-19 and Chloroquine/Hydroxychloroquine: Is there Ophthalmological Concern?Am J Ophthalmol 2020 213:A3-4.10.1016/j.ajo.2020.03.028 [Google Scholar] [CrossRef]
[39]. Tönnesmann E, Kandolf R, Lewalter T, Chloroquine cardiomyopathy-a review of the literatureImmunopharmacol Immunotoxicol 2013 35(3):434-42.10.3109/08923973.2013.78007823635029 [Google Scholar] [CrossRef] [PubMed]
[40]. Fan HH, Wang LQ, Liu WL, An XP, Liu ZD, He XQ, Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus modelChin Med J (Engl) [Internet] 2020 Mar 6 [cited 2020 Jun 23] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7147283/10.1097/CM9.000000000000079732149769 [Google Scholar] [CrossRef] [PubMed]
[41]. Bailly C, Cepharanthine: An update of its mode of action, pharmacological properties and medical applicationsPhytomedicine 2019 62:15295610.1016/j.phymed.2019.15295631132753 [Google Scholar] [CrossRef] [PubMed]
[42]. Khoja S, Huynh N, Warnecke AMP, Asatryan L, Jakowec MW, Davies DL, Preclinical evaluation of avermectins as novel therapeutic agents for alcohol use disordersPsychopharmacology (Berl) 2018 235(6):1697-709.10.1007/s00213-018-4869-929500584 [Google Scholar] [CrossRef] [PubMed]
[43]. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitroAntiviral Res 2020 178:10478710.1016/j.antiviral.2020.10478732251768 [Google Scholar] [CrossRef] [PubMed]
[44]. Tickell-Painter M, Maayan N, Saunders R, Pace C, Sinclair D, Mefloquine for preventing malaria during travel to endemic areasCochrane Database Syst Rev [Internet] 2017 (10)[cited 2020 Jun 23];. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686653/10.1002/14651858.CD006491.pub429083100 [Google Scholar] [CrossRef] [PubMed]
[45]. Kim DE, Min JS, Jang MS, Lee JY, Shin YS, Park CM, Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cellsBiomolecules 2019 9(11):69610.3390/biom911069631690059 [Google Scholar] [CrossRef] [PubMed]
[46]. Gaunt ER, Hardie A, Claas ECJ, Simmonds P, Templeton KE, Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex Real-Time PCR methodJ Clin Microbiol 2010 48(8):2940-47.10.1128/JCM.00636-1020554810 [Google Scholar] [CrossRef] [PubMed]
[47]. Choy KT, Wong AYL, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitroAntiviral Res 2020 178:10478610.1016/j.antiviral.2020.10478632251767 [Google Scholar] [CrossRef] [PubMed]
[48]. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19N Engl J Med [Internet] 2020 Mar 18 [cited 2020 Jun 24]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7121492/ [Google Scholar]
[49]. Hung IFN, Lung KC, Tso EYK, Liu R, Chung TWH, Chu MY, Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trialThe Lancet 2020 395(10238):1695-704.10.1016/S0140-6736(20)31042-4 [Google Scholar] [CrossRef]
[50]. Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infectionsAntiviral Res 2009 82(3):95-102.10.1016/j.antiviral.2009.02.19819428599 [Google Scholar] [CrossRef] [PubMed]
[51]. Sissoko D, Laouenan C, Folkesson E, M’Lebing AB, Beavogui AH, Baize S, Experimental treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A historically controlled, single-arm proof-of-concept trial in GuineaPLoS Med [Internet] 2016 13(3)[cited 2020 Jun 28]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4773183/10.1371/journal.pmed.100206627284977 [Google Scholar] [CrossRef] [PubMed]
[52]. Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, Favipiravir versus Arbidol for COVID-19: A randomised clinical trial [Internet]Infectious Diseases (except HIV/AIDS) 2020 Mar [cited 2020 Jun 28]. Available from: http://medrxiv.org/lookup/doi/10.1101/2020.03.17.2003743210.1101/2020.03.17.20037432 [Google Scholar] [CrossRef]
[53]. Kumagai Y, Murakawa Y, Hasunuma T, Aso M, Yuji W, Sakurai T, Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adultsInt J Clin Pharmacol Ther 2015 53(10):866-74.10.5414/CP20238826308176 [Google Scholar] [CrossRef] [PubMed]
[54]. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitroCell Res 2020 30(3):269-71.10.1038/s41422-020-0282-032020029 [Google Scholar] [CrossRef] [PubMed]
[55]. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, First case of 2019 Novel Coronavirus in the United StatesN Engl J Med 2020 382(10):929-36.10.1056/NEJMoa200119132004427 [Google Scholar] [CrossRef] [PubMed]
[56]. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Compassionate use of remdesivir for patients with severe Covid-19N Engl J Med [Internet] 2020 Apr 10 [cited 2020 Jun 28]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7169476/ [Google Scholar]
[57]. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trialLancet Lond Engl 2020 395(10236):1569-78.10.1016/S0140-6736(20)31022-9 [Google Scholar] [CrossRef]
[58]. Carrillo-Bustamante P, Nguyen THT, Oestereich L, Günther S, Guedj J, Graw F, Determining Ribavirin’s mechanism of action against Lassa virus infectionSci Rep 2017 7(1):1169310.1038/s41598-017-10198-028916737 [Google Scholar] [CrossRef] [PubMed]
[59]. Matthes E, Funk A, Krahn I, Gaertner K, von Janta-Lipinski M, Lin L, Strong and selective inhibitors of hepatitis B virus replication among novel N4-hydroxy- and 5-methyl-beta-L-deoxycytidine analoguesAntimicrob Agents Chemother 2007 51(7):2523-30.10.1128/AAC.00001-0717404006 [Google Scholar] [CrossRef] [PubMed]
[60]. Tong X, Smith J, Bukreyeva N, Koma T, Manning JT, Kalkeri R, Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogensAntiviral Res 2018 149:34-40.10.1016/j.antiviral.2017.11.00429126899 [Google Scholar] [CrossRef] [PubMed]
[61]. Wulan WN, Heydet D, Walker EJ, Gahan ME, Ghildyal R, Nucleocytoplasmic transport of nucleocapsid proteins of enveloped RNA virusesFront Microbiol 2015 6:55310.3389/fmicb.2015.0055326082769 [Google Scholar] [CrossRef] [PubMed]
[62]. Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from ChinaClin Immunol Orlando Fla 2020 214:108393PMID: 3222246610.1016/j.clim.2020.10839332222466 [Google Scholar] [CrossRef] [PubMed]
[63]. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19 [Internet]New England Journal of Medicine. Massachusetts Medical Society 2020 [cited 2020 Jul 5]. Available from: https://www.nejm.org/doi/10.1056/NEJMc2007575. PMID: 30774631 [Google Scholar]
[64]. Crayne CB, Albeituni S, Nichols KE, Cron RQ, The immunology of macrophage activation syndromeFront Immunol 2019 10:11910.3389/fimmu.2019.0011930774631 [Google Scholar] [CrossRef] [PubMed]
[65]. Al-Samkari H, Berliner N, Hemophagocytic LymphohistiocytosisAnnu Rev Pathol Mech Dis 2018 13(1):27-49.10.1146/annurev-pathol-020117-04362528934563 [Google Scholar] [CrossRef] [PubMed]
[66]. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, COVID-19: consider cytokine storm syndromes and immunosuppressionLancet Lond Engl 2020 395(10229):1033-34.10.1016/S0140-6736(20)30628-0 [Google Scholar] [CrossRef]
[67]. Sarzi-Puttini P, Giorgi V, Sirotti S, Marotto D, Ardizzone S, Rizzardini G, COVID-19, cytokines and immunosuppression: What can we learn from severe acute respiratory syndrome?Clin Exp Rheumatol 2020 38(2):337-42. [Google Scholar]
[68]. Chen R, Tang X, Tan S, Liang B, Wan Z, Fang J, Treatment of severe acute respiratory syndrome with glucosteroids: The Guangzhou experienceChest 2006 129(6):1441-52.10.1378/chest.129.6.144116778260 [Google Scholar] [CrossRef] [PubMed]
[69]. Auyeung TW, Lee JSW, Lai WK, Choi CH, Lee HK, Lee JS, The use of corticosteroid as treatment in SARS was associated with adverse outcomes: A retrospective cohort studyJ Infect 2005 51(2):98-102.10.1016/j.jinf.2004.09.00816038758 [Google Scholar] [CrossRef] [PubMed]
[70]. Ye Q, Wang B, Mao J, The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19J Infect 2020 80(6):60710.1016/j.jinf.2020.03.03732283152 [Google Scholar] [CrossRef] [PubMed]
[71]. Tanaka T, Narazaki M, Kishimoto T, Immunotherapeutic implications of IL-6 blockade for cytokine stormImmunotherapy 2016 8(8):959-70.10.2217/imt-2016-002027381687 [Google Scholar] [CrossRef] [PubMed]
[72]. Bevacizumab in severe or critical patients with COVID-19 Pneumonia - Full Text View - ClinicalTrials.gov [Internet]. [cited 2020 Jun 28]. Available from: https://clinicaltrials.gov/ct2/show/NCT04275414 [Google Scholar]
[73]. Leisch M, Egle A, Greil R, Plitidepsin: A potential new treatment for relapsed/refractory multiple myelomaFuture Oncol Lond Engl 2019 15(2):109-20.10.2217/fon-2018-049230111169 [Google Scholar] [CrossRef] [PubMed]
[74]. Landolt L, Furriol J, Babickova J, Ahmed L, Eikrem Ø, Skogstrand T, AXL targeting reduces fibrosis development in experimental unilateral ureteral obstructionPhysiol Rep 2019 7(10):e1409110.14814/phy2.1409131134766 [Google Scholar] [CrossRef] [PubMed]
[75]. Mitchell WM, Efficacy of rintatolimod in the treatment of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME)Expert Rev Clin Pharmacol 2016 9(6):755-70.10.1586/17512433.2016.117296027045557 [Google Scholar] [CrossRef] [PubMed]
[76]. Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, COVID-19: Melatonin as a potential adjuvant treatmentLife Sci 2020 250:11758310.1016/j.lfs.2020.11758332217117 [Google Scholar] [CrossRef] [PubMed]
[77]. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N, Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19Stem Cells Dev 2020 29(12):747-54.10.1089/scd.2020.008032380908 [Google Scholar] [CrossRef] [PubMed]
[78]. Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, Convalescent plasma treatment reduced mortality in patients with severe pandemic Influenza A (H1N1) 2009 virus infectionClin Infect Dis 2011 52(4):447-56.10.1093/cid/ciq10621248066 [Google Scholar] [CrossRef] [PubMed]
[79]. Soo YOY, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KKS, Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patientsClin Microbiol Infect 2004 10(7):676-78.10.1111/j.1469-0691.2004.00956.x15214887 [Google Scholar] [CrossRef] [PubMed]
[80]. van Griensven J, Edwards T, de Lamballerie X, Semple MG, Gallian P, Baize S, Evaluation of Convalescent Plasma for Ebola Virus Disease in GuineaN Engl J Med 2016 374(1):33-42.10.1056/NEJMoa151181226735992 [Google Scholar] [CrossRef] [PubMed]
[81]. Zhou B, Zhong N, Guan Y, Treatment with convalescent plasma for Influenza A (H5N1) infectionN Engl J Med 2007 357(14):1450-51.10.1056/NEJMc07035917914053 [Google Scholar] [CrossRef] [PubMed]
[82]. Chen L, Xiong J, Bao L, Shi Y, Convalescent plasma as a potential therapy for COVID-19Lancet Infect Dis 2020 20(4):398-400.10.1016/S1473-3099(20)30141-9 [Google Scholar] [CrossRef]
[83]. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Effectiveness of convalescent plasma therapy in severe COVID-19 patientsProc Natl Acad Sci USA 2020 117(17):9490-96.10.1073/pnas.200416811732253318 [Google Scholar] [CrossRef] [PubMed]
[84]. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent PlasmaJAMA 2020 323(16):1582-89.10.1001/jama.2020.478332219428 [Google Scholar] [CrossRef] [PubMed]
[85]. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19JAMA 2020 324(5):01-11.10.1001/jama.2020.1004432492084 [Google Scholar] [CrossRef] [PubMed]
[86]. Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibodyEmerg Microbes Infect 2020 9(1):382-85.10.1080/22221751.2020.172906932065055 [Google Scholar] [CrossRef] [PubMed]
[87]. Lutz HU, Späth PJ, Anti-inflammatory effect of intravenous immunoglobulin mediated through modulation of complement activationClin Rev Allergy Immunol 2005 29(3):207-12.10.1385/CRIAI:29:3:207 [Google Scholar] [CrossRef]
[88]. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis [Internet]. [cited 2020 Oct 10]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6178621/ [Google Scholar]
[89]. Abe Y, Horiuchi A, Miyake M, Kimura S, Anti-Cytokine nature of natural human immunoglobulin: One possible mechanism of the clinical effect of intravenous immunoglobulin therapyImmunol Rev 1994 139(1):05-19.10.1111/j.1600-065X.1994.tb00854.x7927413 [Google Scholar] [CrossRef] [PubMed]
[90]. Kulkarni R, Antibody-dependent enhancement of viral infections. In: Bramhachari PV, editorDynamics of Immune Activation in Viral Diseases [Internet] 2020 [cited 2020 Oct 10] SingaporeSpringer:9-41.Available from: https://doi.org/10.1007/978-981-15-1045-8_210.1007/978-981-15-1045-8_231922036 [Google Scholar] [CrossRef] [PubMed]
[91]. Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infectionChest 2020 158(1):e09-13.10.1016/j.chest.2020.03.03932243945 [Google Scholar] [CrossRef] [PubMed]
[92]. Chowdhury FR, Hoque A, Chowdhury FUH, Amin MR, Rahim A, Rahman MM, Convalescent plasma transfusion therapy in severe COVID-19 patients- a safety, efficacy and dose response study: A structured summary of a study protocol of a phase II randomised controlled trialTrials 2020 21(1):88310.1186/s13063-020-04734-z33106167 [Google Scholar] [CrossRef] [PubMed]