Case Report
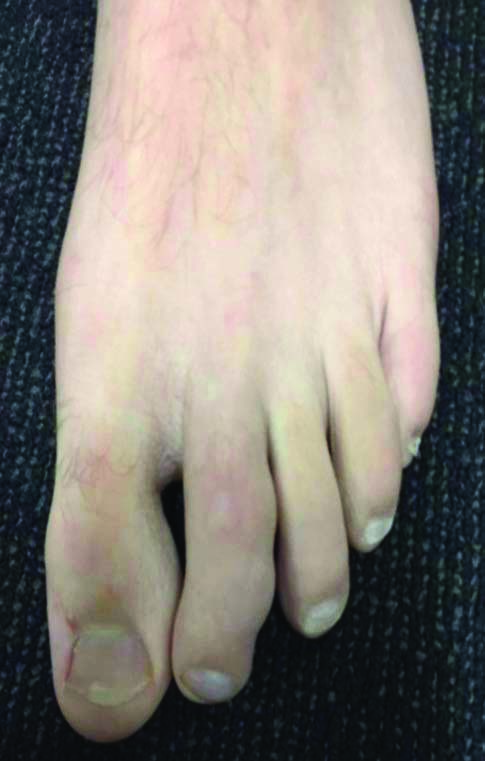
A 16-year-old male, Australian Rules Football (AFL) player was referred to our surgical practice following 18 months of increasing pain and disfigurement of his left second and third toes that was preventing him from participating in sport. His medical history included a ruptured liver in 2017 and concussion in 2018. He did not take any medications and had no allergies or sensitivities. Physical examination revealed three 8 mm circular lesions [Table/Fig-1]. Moderate pain was elicited upon palpation.
Immediate preoperative photographs showing disfigurement of the second toe.
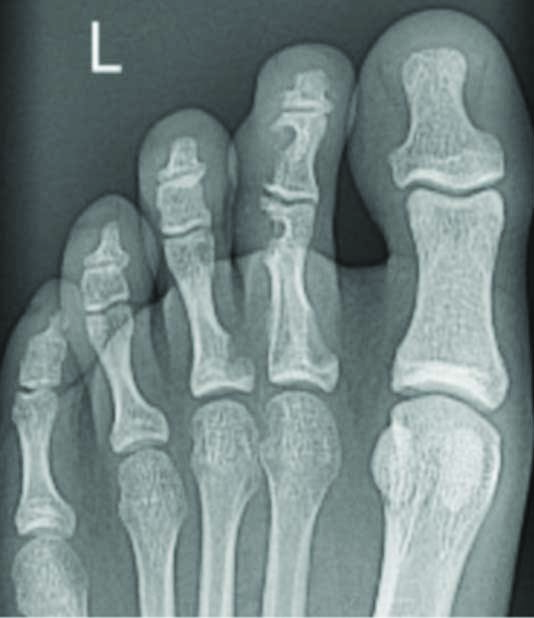
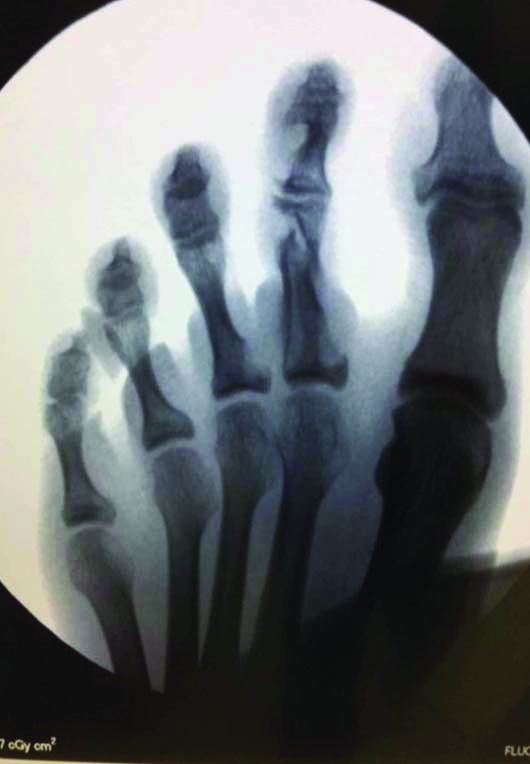
X-Rays [Table/Fig-2] taken one month preoperatively displayed lesions within the second and third toes and an immediate preoperative fluoroscopy exhibited a pathological fracture of the second proximal phalanx [Table/Fig-3].
Anterio-posterior X-rays showed multiple lesions within the left second intermediate and left second and third proximal phalanx.
Immediate preoperative fluoroscopy showing pathological fracturing of the second proximal phalanx.
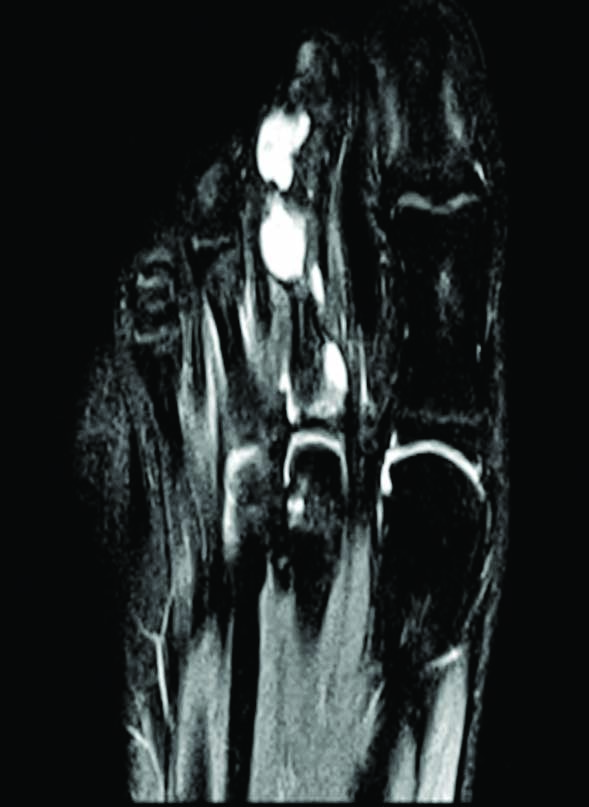
Potential differential diagnoses included osteochondromas, chondrosarcomas, and Hereditary Multiple Exostosis (HME) [1]. MRI showed osteolytic lesions of the left second proximal and middle phalanx and the proximal phalanx of the third toe [Table/Fig-4].
Magnetic Resonance Imaging (MRI) showing multiple lucent lesions.
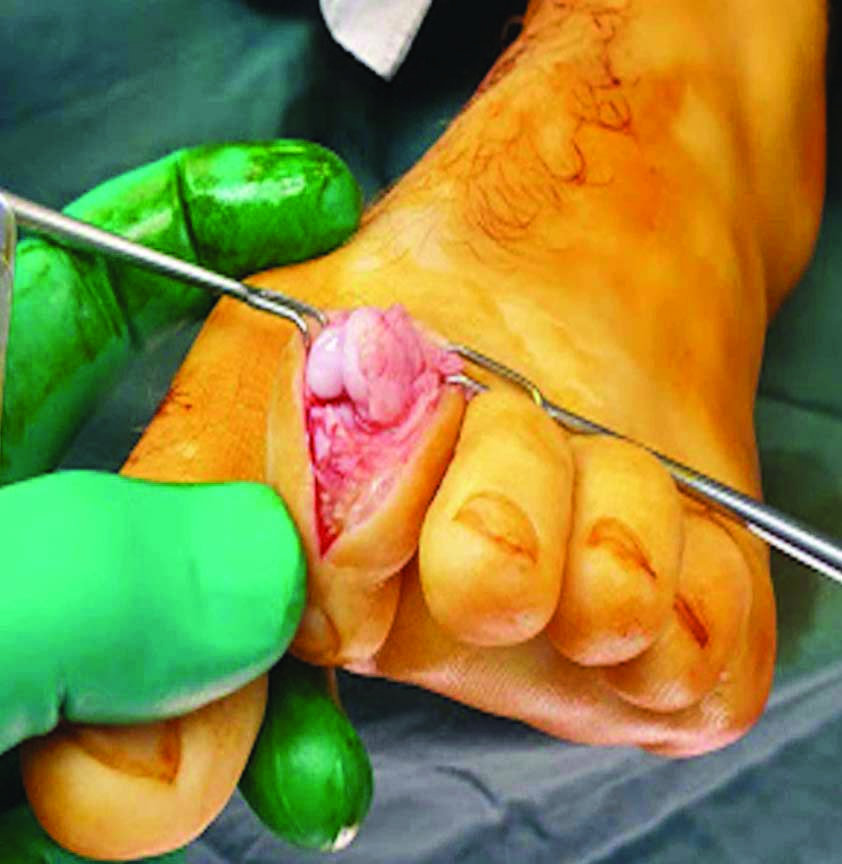
The patient’s left foot was prepped and draped in the usual fashion to facilitate an aseptic field. A calf tourniquet was inflated to 250 mmHg. A 15 mL 0.75% ropivacaine hydrochloride mixed with 4 mg dexamethasone sodium phosphate was administered just proximal to the second and third metatarsophalangeal joints. Dorsal linear incisions were employed over the second and third digits. All lesions were debrided with a small bone curette [Table/Fig-5] with fenestration using a single 1.6 mm Kirshner wire to promote bleeding. Bone graft was harvested from the ipsilateral tibia and inserted into each cavity left by the evacuated lesions. Two percutaneous Kirschner wires [Table/Fig-6] were employed to stabilise the second toe postoperatively.
An enchondroma within the chondral plate.
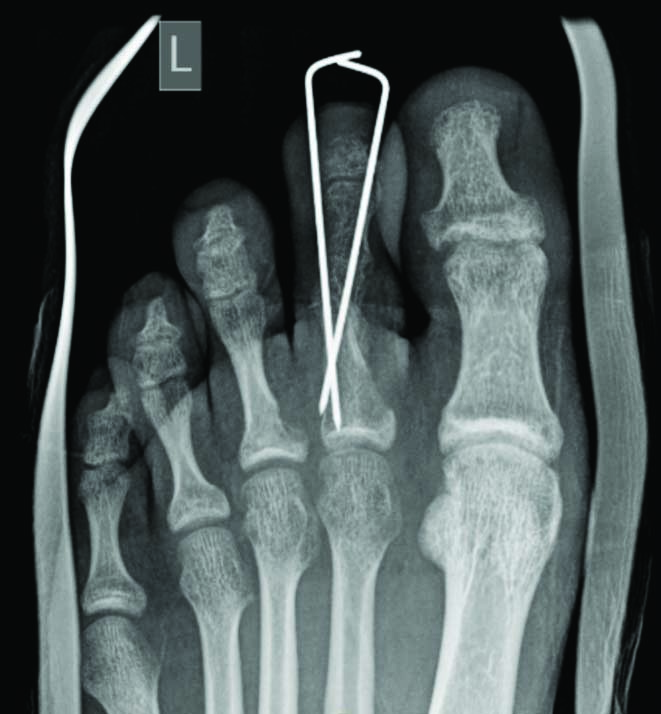
Two Kirshner wires were employed to stabilise the second digit for six weeks postoperatively.
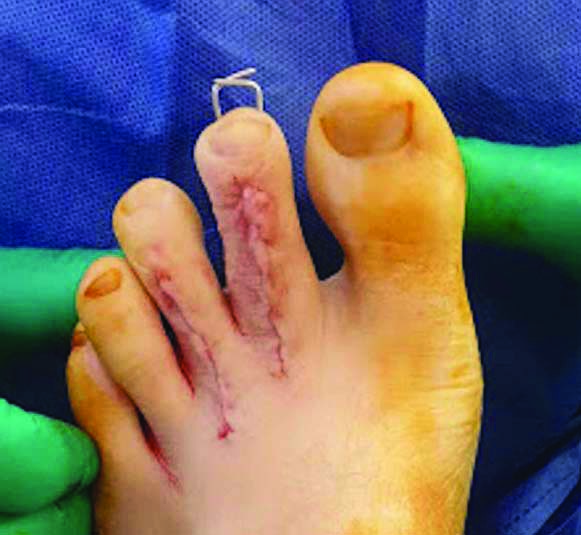
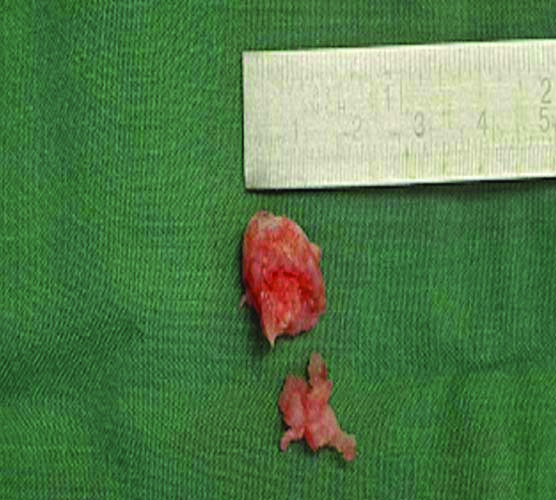
Multi-modal treatment methods were implemented including careful surgical technique and preparation of wound edges, even wound approximation with minimal tension, careful resection and insertion of the autograft fragments, and postoperative antibiotics with antiseptic dressings to reduce the risk of infection. Early compression therapy was employed. The specimens [Table/Fig-7] were sent for histology, confirming the diagnosis of enchondromatosis [Table/Fig-8]. He was allowed to weight-bear immediately but remained in his postoperative shoe for six weeks. At six weeks postoperatively, X-rays were taken to confirm osseous consolidation [Table/Fig-9] and the Kirschner wires were removed [Table/Fig-10]. At this appointment the patient reported no pain and his toes were no longer deformed and in normal alignment. This continued up until his discharge appointment at 12-months postoperatively.
Two enchondromas. The larger fragment was resected from the second proximal phalanx and the smaller from the second middle phalanx.
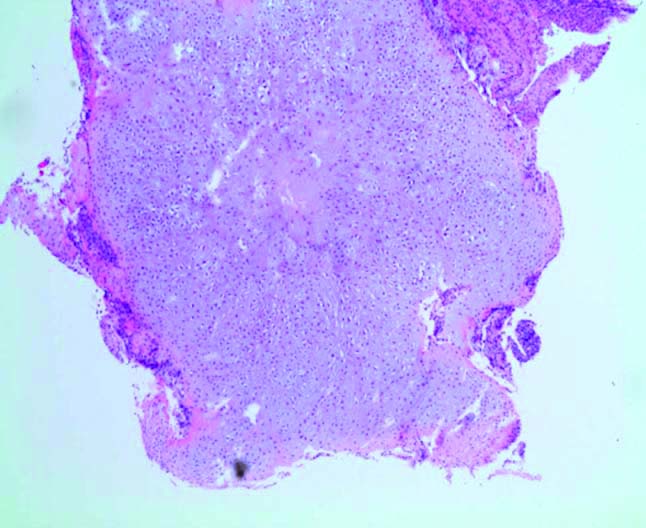
The enchondroma micrograph. The fragments were submitted for decalcification before processing and then processed as four transverse slices in two blocks. Haematoxylin & Eosin staining at 1.25x magnification showed well differentiated cartilaginous lesions comprising a lobulated proliferation of hypercellular chondroid tissue.
Radiograph taken six weeks postoperatively immediately before Kirshner wire removal.
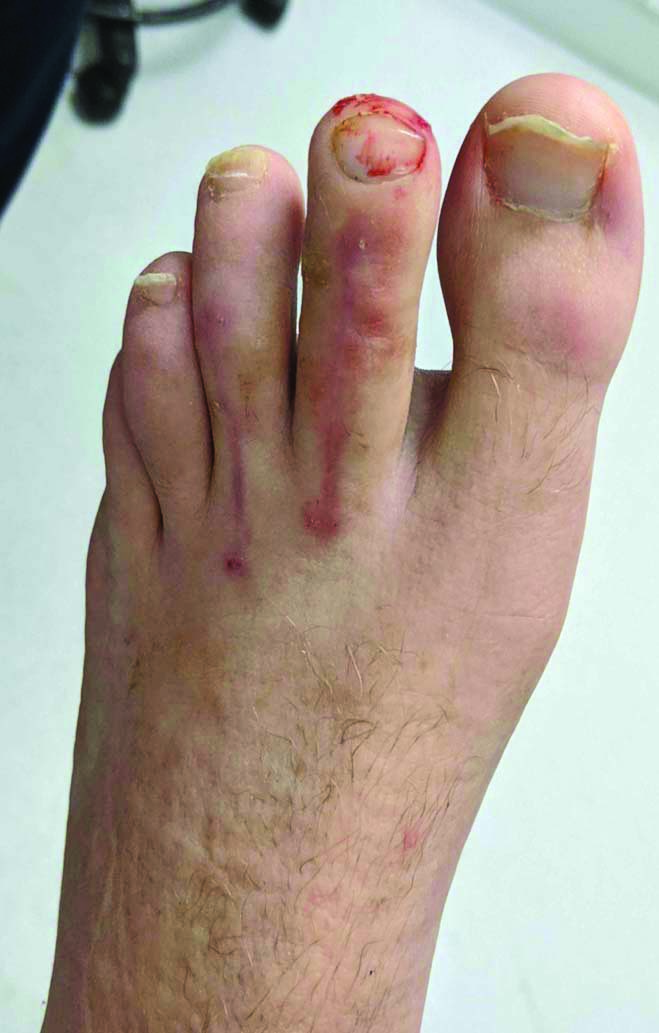
Clinical picture six weeks postoperatively immediately after Kirshner wire removal.
Discussion
Enchondromas are the most common tumours of the foot bones after osteochondromas and are the most common tumours in the long bones of the hands. When multiple enchondromas are present the condition is called enchondromatosis, best known by the eponym Ollier disease, named after the physician who described it in 1898. In the presence of additional soft tissue haemangiomas, it is called Maffucci syndrome. The lesions of enchondromatosis arise from cartilage cell nests that have fragmented from the central physis during the first to fourth decades of life. The prevalence of enchondromatosis is estimated to be 1/100,000 [1]. Enchondromatosis is characterised by asymmetrical cartilage lesions of variable distribution that cause pain, deformity, changes to limb length, and potential malignancy [2,3].
Enchondromas are benign cartilaginous tumours arising within long bones. When multiple lesions are present it is termed enchondromatosis. This condition may result in bony masses causing deformity and bone shortening, broadening, bending or curving [1] and usually affects the tibia, femur, phalanges, ilium or flat bones. The association of multiple enchondromas with haemangiomas is referred to as Maffucci syndrome [1]. The pain associated with enchondromatosis is due to increasing pressure of the expansion of the lesions on the surrounding bone [4], the pathologic fracture of the surrounding cortices, malignant conversion, and the gradual enlargement of the digits [1]. Enchondromas present as lytic lesions with well-defined borders and varying degrees of punctate calcifications [4] without the soft-tissue involvement and indeterminate borders characteristic of chondrosarcomas [1].
Enchondromatosis may lead to malignancy, with the rate of transformation reported between 20% to 50% [1]. A subtype-central chondrosarcoma- located within the medullary cavity, may lead to sarcomatous change in an underlying enchondroma. This malignant degeneration appears greater in Maffucci syndrome [5]. The cause of enchondromatosis is unknown and it seems to occur spontaneously. It is considered non-hereditary, yet there is some evidence that it may be an autosomal-dominant genetic trait [5]. The estimated prevalence of enchondromatosis is reported to be 1 in 100,000 [5], yet the true incidence may be higher, as mild phenotypes without skeletal deformities may go undetected [6-8].
At the end of the 19th century, Ollier emphasised the asymmetrical and random distribution of enchondromas [1]. Most often thought of as the same condition, some authors [6] distinguish between enchondromatosis and Ollier disease. The former affecting mostly men and characterised by enchondromas located in the extremities transmitted in an autosomal dominant fashion. The latter affects mostly women and is characterised by the sporadic unilateral distribution of enchondromas. Nevertheless, this two-form classification is not yet accepted. Some authors have further progressed this classification system [7]. Recently, a previously unreported form in which there is extensive involvement of the epiphyseal and metaphyseal regions of long bones of the lower extremity has been described [7].
Enchondromatosis is a spontaneous, non-familial disorder [8-10]. Two case reports [11,12], reported male patients with enchondromatosis whose fathers had mild skeletal dysplasia. The irregular distribution of lesions in these cases were similar to present case (our patient also had a prior calcaneal enchondroma treated surgically). This variant of enchondromatosis may indicate an endochondral bone formation disorder resulting from a postzygotic somatic mutation.
Interestingly, a mutant Parathyroid Hormone 1 (PTHR 1) (R150C) genome was expressed in the enchondromas of one-third of unrelated patients with enchondromatosis [11]. This mutation was found in a parental allele in one patient whose father had atypical skeletal dysplasia. Spranger J et al., could not identify the R150C mutation (26 tumours) nor mutation of the PTHR1 gene (11 patients), suggesting heterogeneity of the molecular defect(s). Little is known about the mechanisms of malignant transformation of enchondromatosis. Four groups of researchers [13-16] reported that the expression of the genes PTHrP, the PTHR1 along with their downstream partner B-cell Lymphoma-2 (BCL-2) may be correlated with malignant transformation to chondrosarcoma, however this remains speculative.
Diagnosis of enchondromatosis is based on clinical and radiological evaluations. Differential diagnoses may include osteochondromas, chondrosarcomas, and HME [1]. This differentiation between enchondromas and chondrosarcomas is important. Mirra JM et al., reported that patients with chondrosarcoma were 12-year older than those with enchondroma and that 97% of patients with a chondrosarcoma reported pain, compared to only 44% with enchondromas [17]. Murphy MD et al., reported deep endosteal scalloping, cortical destruction, soft tissue involvement and periosteal reactions were classical of chondrosarcoma [18].
Enchondromatosis presents with well-defined lucent lesions within the long bones on X-ray [1]. As the lesions expand they place pressure on the surrounding bone resulting in cortical thinning, which may lead to bone stress and fracture [1]. Diagnostic Ultrasonography (US) may show soft tissue oedema, and MRI may exhibit lucent expansile lesions. Radiographically, enchondromas exhibit sharp, well-delineated borders compared with the irregular margins of chondrosarcomas.
The lesions of enchondromatosis are similar to cartilage [Table/Fig-9], with comparable amounts of chondrocytes spread in hyaline matrix lacunae [2]. Structurally, they are nodular with connective tissue septa and calcifications. Phalangeal enchondromatosis lesions exhibit some mitotic activity, yet are benign [1-3]. Usually, chondrosarcoma and enchondroma are microscopically similar, with the presence of bi-nucleated chondrocytes required to diagnose a chondrosarcoma [18]. Enchondroma lobules are regular and consist of fibrous connective tissue, whereas chondrosarcoma have irregular lobules with cellular fibrous tissue encircling the tumour [18]. Histopathology of our specimens showed well-circumscribed lobules of hyaline cartilage encased by bone and covered with fibrous tissue.
Our specimens showed well-differentiated cartilaginous lesions composed of lobulated hypercellular chondroid tissue. The lobules were separated by fibrous septa containing delicate vessels. The chondrocytes had small pyknotic, stellate and ovoid hyperchromatic nuclei and surrounding pale cytoplasm. No mitotic features, definite cortical bone erosion or lamellar bone entrapment were represented. There were no sarcomatoid areas or other features of a high grade malignancy. Overall, the specimens were reported as well-differentiated chondroid neoplasms consistent with enchondromatosis.
There is no consensus treatment for enchondromatosis. Kumar A et al., recommended periodic surveillance of the brain and abdomen for occult lesions in patients with enchondromatosis [19]. Surgery is indicated in the case of structural complications like fractures, growth defects and malignant change.
Tang C et al., reviewed the outcomes of curettage, curettage without augmentation, curettage with augmentation by bone graft, and curettage with augmentation by cement injection for the treatment of hand enchondromas [20]. They reported high rates of non-union with curettage in isolation (67%) and using cement augmentation did not improve outcomes. The group who underwent augmentation with bone graft enjoyed the best results with a similar recovery time to isolated curettage, albeit with the inevitable additional wound that required healing [18]. The authors recommended 6-monthly radiographic checks for small asymptomatic enchondromas, that questionable lesions be graded by the Enneking staging system and that biopsies be taken when there is a question of diagnosis [1].
The prognosis of enchondromatosis is difficult to assess [20]. Interestingly, patients with numerous lesions may have a better prognosis than those with localised changes, as lesions confined to one region may induce shortening of the extremity resulting in limb asymmetry. The early onset of the disease appears to worsen outcomes, especially if the disease begins in puberty. The early development of enchondromas in the fingers or toes may lead to major digital deformities.
Conclusion(s)
Enchondromatosis may result in stress and fracture of bone as well as disfigurement. Debridement of the enchondromas and insertion of calcaneal bone graft can provide reliable symptomatic relief. In this case report, diagnosis was based on radiographic and histopathology results. Close postoperative monitoring was implemented. The patient reported the complete resolution of his symptoms by eight weeks postoperatively which continued up until his discharge at 12 months.
[1]. Gajewski DA, Burnette JB, Murphey MD, Temple HT, Differentiating clinical and radiographic features of enchondroma and secondary chondrosarcoma in the footFoot Ankle Int 2006 27:240-44.10.1177/10711007060270040316624212 [Google Scholar] [CrossRef] [PubMed]
[2]. Landry MM, Sarma DP, In-situ chondrosarcoma of the foot arising in a solitary enchondromaJ Foot Surg 1990 29:324-26. [Google Scholar]
[3]. Murari TM, Callaghan JJ, Berrey BH, Sweet DE, Primary benign and malignant osseous neoplasms of the footFoot Ankle 1989 10:68-80.10.1177/1071100789010002052807109 [Google Scholar] [CrossRef] [PubMed]
[4]. Silve C, Jüppner H, Ollier diseaseOrphanet Journal of Rare Diseases 2006 1:01-06.10.1186/1750-1172-1-3716995932 [Google Scholar] [CrossRef] [PubMed]
[5]. Spranger J, Kemperdieck H, Bakowski H, Optiz JM, Two peculiar types of enchondromatosisPediatr Radiol 1978 7:215-19.10.1007/BF02386711733398 [Google Scholar] [CrossRef] [PubMed]
[6]. Carbonell JM, Vineta TJ, A further case of congenital generalized dyschondrotheosis, Ollier typeRev Esp Pediatr 1962 18:91-99. [Google Scholar]
[7]. Lamy M, Aussannaire M, Jammet ML, Nezelof J, Three cases of Olliers disease in one familyBull Mem Soc Med Hop Paris 1954 70:62-70. [Google Scholar]
[8]. Pannier S, Legeai-Mallet L, Hereditary multiple hereditary exostoses and enchondromatosisBest Pract Res Clin Rheumatol 2008 22:45-54.10.1016/j.berh.2007.12.00418328980 [Google Scholar] [CrossRef] [PubMed]
[9]. Unni KK, Cartilaginous lesions of boneJ Orthop Sci 2001 6:457-72.10.1007/s00776017001511845358 [Google Scholar] [CrossRef] [PubMed]
[10]. Whyte M, Acquired disorders of cartilage and bone 2003 Washington DCAmerican Society for Bone and Mineral Research [Google Scholar]
[11]. Halal F, Azouz EM, Generalized enchondromatosis in a boy with only platyspondyly in the fatherAm J Med Genet 1991 38:588-92.10.1002/ajmg.13203804182063903 [Google Scholar] [CrossRef] [PubMed]
[12]. Hopyan S, Gokgoz N, Poon R, Gensure RC, Yu C, Cole WG, A mutant PTH/ PTHrP type I receptor in enchondromatosisNat Genet 2002 30:306-10.10.1038/ng84411850620 [Google Scholar] [CrossRef] [PubMed]
[13]. Amling M, Posl M, Hentz MW, Priemel M, Delling G, PTHrP and Bcl- 2: Essential regulatory molecules in chondrocyte differentiation and chondrogenic tumorsVerh Dtsch Ges Pathol 1998 82:160-69. [Google Scholar]
[14]. Bovee JV, van den Broek LJ, Cleton-Jansen AM, Hogendoorn PC, Upregulation of PTHrP and Bcl-2 expression characterises the progression of osteochondroma towards peripheral chondrosarcoma and is a late event in central chondrosarcomaLab Invest 2000 80:1925-34.10.1038/labinvest.378020211140704 [Google Scholar] [CrossRef] [PubMed]
[15]. Kunisada T, Moseley JM, Slavin JL, Martin TJ, Choong PFM, Co-expression of parathyroid hormone-related protein (PTHrP) and PTH/PTHrP receptor in cartilaginous tumours: A marker for malignancy?Pathology 2002 34:133-37.10.1080/00313020120111793612009094 [Google Scholar] [CrossRef] [PubMed]
[16]. Pateder DB, Gish MW, O’Keefe RJ, Teot LA, Rosier RN, Parathyroid hormone-related Peptide expression in cartilaginous tumorsClin Orthop 2002 403:198-204.10.1097/00003086-200210000-0002912360027 [Google Scholar] [CrossRef] [PubMed]
[17]. Mirra JM, Gold R, Downs J, Eckart JJ, A new histologic approach to the differentiation of enchondroma and chondrosarcoma of the bones. A clinicopathologic analysis of 51 casesClin Orthop Relat Res 1985 201:214-37.10.1097/00003086-198512000-00035 [Google Scholar] [CrossRef]
[18]. Murphy MD, Flemming DJ, Boyea SR, Bojescul JA, Sweet DE, Temple HT, Enchondroma versus chondrosarcoma in the appendicular skeleton: Differentiating featuresRadiographics 1998 18:1213-37.10.1148/radiographics.18.5.97476169747616 [Google Scholar] [CrossRef] [PubMed]
[19]. Kumar A, Jumar Jain V, Bharadwaj M, Arya RK, Ollier disease: Pathogenesis, diagnosis, and managementOrthopedics 2015 38:497-506.10.3928/01477447-20150603-5826091223 [Google Scholar] [CrossRef] [PubMed]
[20]. Tang C, Chan M, Fok M, Fung B, Current management of hand enchondroma: A reviewHand Surg 2015 20:191-95.10.1142/S021881041530002825609298 [Google Scholar] [CrossRef] [PubMed]