Granulosa Cell Tumour (GCT) is a rare malignant ovarian tumour. Adult GCT variety is more common than juvenile variety and occurs usually in postmenopausal women. It showed a spectrum of the imaging findings due to various histological appearances. It may present with solid masses, multilocular cystic lesion or completely cystic lesion. Here, authors present an interesting case of a 27-year-old young female, who presented with a large lump in the abdomen. On Magnetic Resonance Imaging (MRI) there was a large well-defined, multilocular cystic lesion at superior aspect of the lesion and complex cystic, solid mass at inferior aspect. Functional MRI like diffusion weighted imaging which provides good image contrast helped in determining the malignancy despite a benign diagnosis on ultrasound guided biopsy and furthering the patient for histopathological examination to come to a final diagnosis.
Case Report
A 27-year-old female patient was referred to the Department of Radiology for ultrasound with complaints of a large firm immobile abdominal lump since one and half years and lower back ache since six months. The patient also had a history of irregular menses for the past two years. She was a mother of a six-year-old healthy female child at the time of presentation. No co-morbidities were present. The lump was smaller earlier however, showed progressive increase in size over a period of six months. No history of carcinoma in the family at this time was known.
On clinical evaluation, the abdomen showed distension with a hard round palpable mass in the midline almost occupying the entire abdomen till the epigastrium superiorly. The lower margin of the mass could not be ascertained on palpation. No tenderness was observed. However, the patient experienced dull pain in the bilateral flanks.
Ultrasound abdomen and pelvis was performed, which revealed a large multiloculated cystic mass measuring approximately 20 (Craniocaudal) × 12 (Transverse) × 10 (Anteroposterior) cm which extended from the right hypochondrium region to the pelvis. There were internal echoes and multiple septae seen in the mass. Uterus was normal in size and echotexture, however mildly displaced to the right side. Myometrial thickness was normal. Both ovaries were not clearly visualised. MRI Pelvis with contrast was advised for the further evaluation.
The MRI was conducted using a 3-T MR system with a phased array coil (Magnetom Vida, Siemens, and Erlangen, Germany). Axial T1WI (T1-Weighted Image), T2WI, T2 STIR, and sagittal T2WI and coronal T1WI, T2 STIR were included in the protocols used. Diffusion Weighted Imaging (DWI) using an Echo Planar Imaging Two-Dimensional (EP2D) sequence performed in the axial plane with parallel acquisition technique by using b-value=0, 50 and 800 s/mm. Dynamic contrast-enhanced imaging was done using T1 VIBE TWIST DIXON.
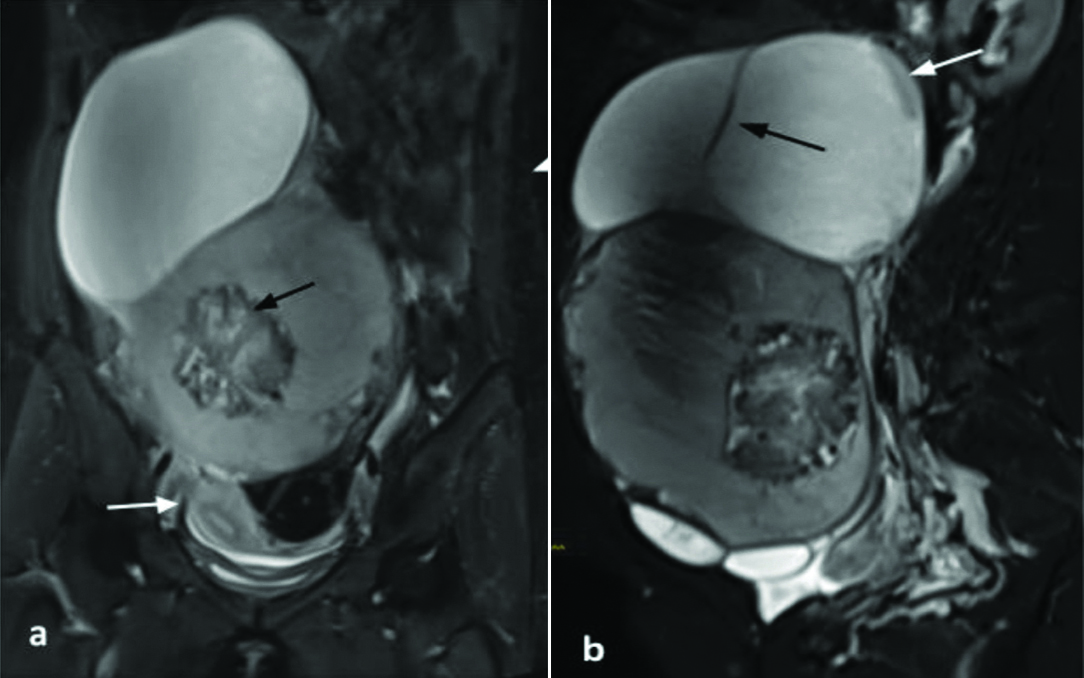
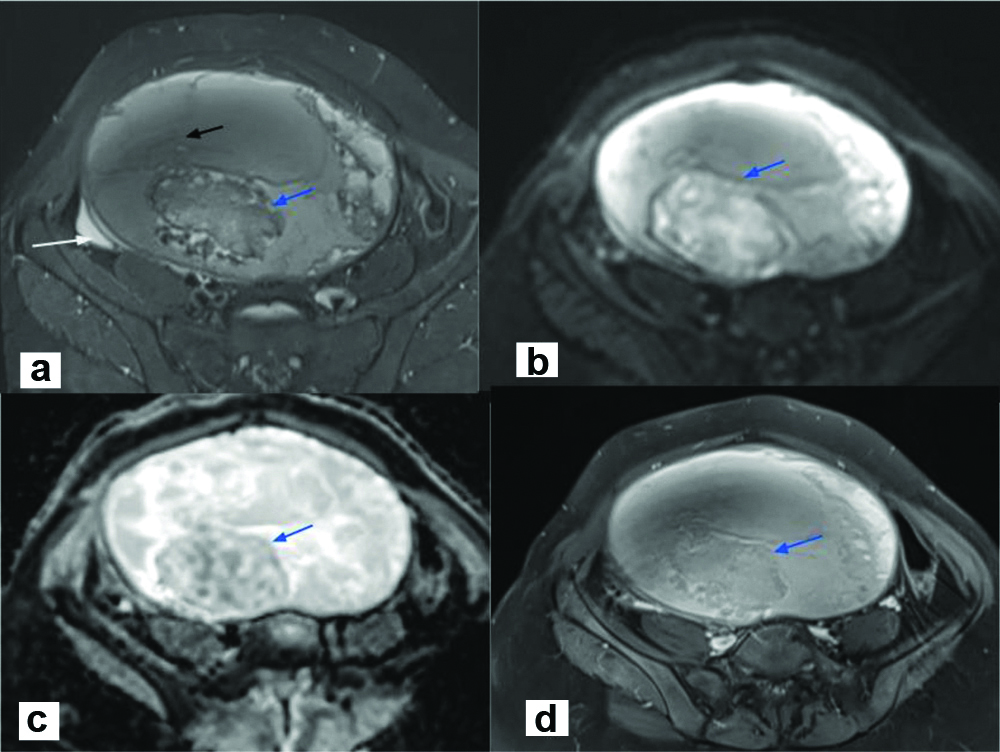
The MRI revealed a large well defined multiloculated cystic lesion occupying the pelvis; superiorly it was reaching upto the right hypochondrium and epigastrium. Mass was measured approximately 23.6 cm (craniocaudal) × 16 cm (transverse) × 10 cm (anteroposterior) in size It was displacing the uterus inferiorly and towards the right side [Table/Fig-1]. The lesion was mainly divided into two compartments- superior and inferior, differing in the nature of their fluid content. The superior portion of the mass appeared hypointense on T1WI and hyperintense on T2WI suggestive of serous fluid. However, the inferior portion appeared hyperintense on T1WI and showed intermediate signal intensity on T2WI, most likely suggestive of serous and haemorrhagic contents. The lesion showed multiple thick irregular enhancing septations some of which also showed nodular deposits/thickening. The inferior compartment showed a solid component within, which measured approximately 7.5 cm (craniocaudal) × 7.2 cm (transverse) × 5.2 cm (anteroposterior). This solid component showed heterogeneous signal intensity on T1WI, T2WI and STIR [Table/Fig-2a]. Few haemorrhagic foci also observed in the mass. The solid component showed patchy diffusion restriction on DWI with corresponding low Apparent Diffusion Coefficient (ADC) values [Table/Fig-2b,c]. Mild postcontrast enhancement, more in the periphery, was noted in the solid component and in few of the septae [Table/Fig-2d]. The right ovary was not separately seen. The left ovary was seen and was normal with multiple small follicles. The uterus was also normal. Minimal free fluid was seen in the pelvis. The bowel loops were displaced laterally. No peritoneal seeding was seen. Enlarged lymph nodes were not present. The peculiarities like young age of the patient, thick enhancing septae, enhancing solid component were highly suggestive of ovarian malignancy. Biopsy was thus suggested.
a) STIR coronal MRI image showed a large cystic mass occupying the abdomen and pelvis. It has displaced uterus inferior and on right side (white arrow). Inferior cystic lesion showed a solid mass (black arrow); b) STIR sagittal MRI image showed both cystic lesions showed different signal intensity and multiple septae also visualised (black arrow) along with nodular thickening (white arrow).
a) STIR Axial image showed the complex solid cystic mass with multiple septations (black arrow) and solid component (blue arrow), minimal free fluid (white arrow); b) Solid mass showed few areas of Diffusion restriction (blue arrow); c) Showing low ADC values.(blue arrow); d) solid mass showed mild postcontrast enhancement (blue arrow).
Ultrasound guided biopsy of the mass was performed, and histopathology was reported as benign serous cystadenoma. But tumour markers showed that Inhibin B levels were markedly raised and were 424 ng/dL. Other markers like Cancer Antigen 125 (CA-125), Human Chorionic Gonadotropin (HCG), Lactate Dehydrogenase (LDH) and Alfa-Fetoprotein (AFP) were within normal range. So, the risk malignancy index was calculated as ultrasound score×menopausal score×tumour marker (3×1×424) which was high. With the provisional diagnosis of malignant ovarian tumour she was taken up for explorative laparotomy. Postsurgical histopathology of the solid tumour component revealed that it was malignant GCT of the right ovary (Stage 1C).
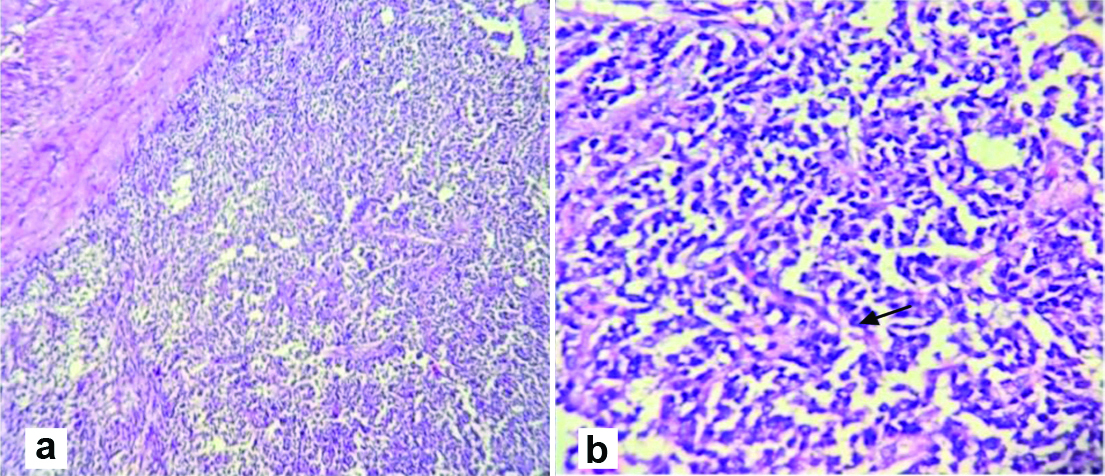
Histopathology observation of the surgical specimen on microscopic examination revealed fibrocollagenous cyst wall lined by tumour cells arranged in sheets, cords, trabeculae, nests and at places, arranged in follicles. The cells have scanty, pale cytoplasm and round to oval nuclei, few showing nuclear grooves. Mitotic activity is not noted. The surrounding tissues shows fibromatous and thecomatous stroma. There are large areas of haemorrhage and haemosiderin laden macrophages in a few sections. A small area of necrosis is noted surrounded by foamy histiocytes [Table/Fig-3]. Section 10 shows an unremarkable wall of fallopian tube. The tumour was positive for immunomarker calretinin (90%) and WT-1 (50%). It was CD-10 and ER/PR negative. It was concluded to be a right ovarian tumour showing features of GCT.
Histopathology of the mass revealed fibrocartilagenous cyst wall lined by tumour cells arranged in sheets, cords, trabeculae, nests and at places, arranged in follicles shown in H and E stain, 100x and 400x magnification.
The patient tolerated the surgery well and was advised to follow-up. Patient was well on the postsurgery follow-up after six weeks and three months clinically.
Discussion
Granulosa Cell Tumour (GCT) of the ovary is a rare but the most common (70%) ovarian sex cord stromal tumour that makes up only 2-3% of all ovarian malignancies [1]. It is also the most common oestrogenic or hormone producing (80%) ovarian tumour [2,3]. Its incidence is merely 0.5-1.5 per 100,000 women per year [4]. One-third of GCTs occur in premenopausal women, more than 50% in postmenopausal women and 5% in the prepubertal period. Pathologically, Ovarian Granulosa Cell Tumour (OGCT) are classified into two subtypes: adult (95%) and juvenile form (5%) [2].
Abnormal vaginal bleeding may occur and it can be associated with endometrial hyperplasia, hyperestrogenic state polyps, and carcinoma (3-25% of cases). Prognosis is usually excellent but correlates with stage and age at the time of the diagnosis (>90% have a 10-year survival rate at stage I) [1].
The GCTs are sex cord stromal tumours with low malignant potential despite a favourable prognosis because of 20-30% cases showing metastasis or recurrence [5]. They are slow growing and have a tendency of late recurrence that is 10-20 years after diagnosis. Blood borne or lymph node metastasis are also rare but cases of pulmonary, skeletal and splenic metastasis are also reported [6-8]. Chemotherapy is recommended as adjuvant to surgery for patients with stages II-IV tumour. Overall, 75% of the GCTs are diagnosed as Stage I, 20% as Stage II, 8% as Stage III and 6% as Stage IV [9].
Knowledge of imaging of this rare ovarian tumour is limited. MRI is commonly used as a problem-solving modality in the evaluation of complex adnexal masses indeterminate in Ultrasonography (USG) or Computed Tomography (CT) due to excellent soft-tissue resolution and zero radiation [10]. Owing to variable histologic appearances and diverse configurations of tumour cells, adult GCT exhibits a variety of imaging manifestation. They can be solid masses, tumours with haemorrhagic or fibrotic changes, multilocular cystic lesions or completely cystic tumours. Multilocular cystic appearances are produced by a predominantly macro-follicular pattern of GCT, and these cysts are filled with serous or haemorrhagic fluid. Heterogeneity within a solid tumour is caused by intratumoral haemorrhage, infarction, fibrous degeneration and irregularity in tumour cell arrangement. In comparison to the more common epithelial tumours, at the time of diagnosis, GCT are limited to the ovary with less peritoneal seeding propensities and are rarely bilateral. They can rupture, resulting in haemoperitoneum [11,12].
Kim SH and Kim SH, reported that in their seven cases, tumour haemorrhage was a frequent MRI finding [12]. This feature may be useful in distinguishing between OGCT and Other Sex Cord Stromal Tumour (OSC) since it is not noted in the latter [12].
Zhang H et al., in their study of MR findings of 18 cases of primary ovarian GCT reported that no lymphadenopathy was detected on MRI in these cases, which means it is a low potential malignant tumour unlike ovarian epithelial carcinoma. Even though the type of augmentation between OGCT and OSC did not differ considerably, it may be helpful to differentiate against large ligament fibroids because they often display a marked enhancement on the administration of contrast. MRI could provide useful data to distinguish malignant tumours from benign processes as a problem-solving method [13]. In a recent review, it was also concluded that due to the low lymph node metastasis rate, lymphadenectomy was not recommended in initial staging surgery for ovarian sex cord stromal tumour [14].
DWI is known as a functional imaging technique, by showing random motion of water molecules, enabling the quantitative evaluation of tumour tissues with ADC value. The movement of water molecules in cancer tissues is restricted on DWI images as a result of high cell densities and abundant cell membranes, and therefore the derived ADC value should typically be lower in malignant diseases than in benign or stable tissue [10]. Another advantage that DWI has is that it eliminates the need for intravenous contrast administration for patients suffering from renal impairment. Several studies have indicated that the ADC value could help differentiate between ovarian malignant lesions and benign conditions in both the 1.5 T and 3.0 T MR systems [13,14]. Sometimes, OGCT needs to be differentiated with fibrothecoma and ligamental myoma on MRI. In present case with the help of DWI imaging and the solid mass showed diffusion restriction, so possibility of malignancy was addressed, which was helpful in reaching the final diagnosis.
Conclusion(s)
Ovarian GCT is a low malignant potential tumour and generally does not show seeding or metastasis or lymphadenopathy despite its growing size and stage. With biopsy sometimes resulting in false negative due to the difference in contents of the cysts/loculations constituting the tumour, due importance should be given to imaging features like a solid component showing postcontrast enhancement and restricted diffusion on DW-MRI, thick irregular walls or septae (>3 mm), significant growth in short period of time if present, other concurrent findings like ascites as in this case.
[1]. Jung SE, Rha SE, Lee JM, Park SY, Oh SN, Cho KS, CT and MRI findings of sex cord-stromal tumor of the ovaryAm J Roentgenol 2005 185(1):207-15.10.2214/ajr.185.1.0185020715972425 [Google Scholar] [CrossRef] [PubMed]
[2]. Young RH, Dickersin GR, Scully RE, Juvenile granulosa cell tumor of the ovary: A clinicopathological analysis of 125 casesAm J Surg Pathol 1984 8:575-96.10.1097/00000478-198408000-000026465418 [Google Scholar] [CrossRef] [PubMed]
[3]. Cohen DJ, Ovary and adnexaIn Gynecologic, Obstetric and Breast Radiology, Thurmond AS, Jones MK, Cohen DJ (eds) 1996 Cambridge, MABlackwell Science:255-322. [Google Scholar]
[4]. Malmstrom H, Hogberg T, Risberg B, Simonsen E, Granulosä cell tumors of the ovary: Prognostic factors and outcomeGynecol Oncol 1994 52:50-55.10.1006/gyno.1994.10108307501 [Google Scholar] [CrossRef] [PubMed]
[5]. Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN, Pathology and classification of ovarian tumorsCancer 2003 97(S10):2631-42.10.1002/cncr.1134512733128 [Google Scholar] [CrossRef] [PubMed]
[6]. Sehouli J, Drescher F, Mustea A, Elling D, Friedmann W, Kuhn W, Nehmzow M, Granulosa cell tumor of the ovary: 10 years follow-up data of 65 patientsAnticancer Res 2004 24:1223-30. [Google Scholar]
[7]. Rose PG, Piver MS, Tsukada Y, Lau T, Metastatic patterns in histologic variants of ovarian cancer. An autopsy studyCancer 1989 64:1508-13.10.1002/1097-0142(19891001)64:7<1508::AID-CNCR2820640725>3.0.CO;2-V [Google Scholar] [CrossRef]
[8]. Thirumala SD, Putti TC, Medalie NS, Wasserman PG, Skeletal metastases from a granulosa-cell tumor of the ovary: Report of a case diagnosed by fine-needle aspiration cytologyDiagn Cytopathol 1998 19:375-77.10.1002/(SICI)1097-0339(199811)19:5<375::AID-DC13>3.0.CO;2-O [Google Scholar] [CrossRef]
[9]. Evans AT, Gaffey TA, Malkasian GD, Annegers JF, Clinicopathologic review of 118 granulosa and 82 theca cell tumorsObstet Gynecol 1980 55:231-38. [Google Scholar]
[10]. Sala E, Rockall A, Rangarajan D, Kubik-Huch RA, The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvisEur J Radiol 2010 76(3):367-85.10.1016/j.ejrad.2010.01.02620810230 [Google Scholar] [CrossRef] [PubMed]
[11]. Jung SE, Lee JM, Rha SE, Byun JY, Jung JI, Hahn ST, CT and MR imaging of ovarian tumors with emphasis on differential diagnosisRadiographics 2002 22:1305-25.10.1148/rg.22602503312432104 [Google Scholar] [CrossRef] [PubMed]
[12]. Kim SH, Kim SH, Granulosa cell tumor of the ovary: Common findings and unusual appearances on CT and MRJ Comput Assist Tomogr 2002 26:756-61.10.1097/00004728-200209000-0001612439311 [Google Scholar] [CrossRef] [PubMed]
[13]. Zhang H, Zhang H, Gu S, Zhang Y, Liu X, Zhang G, MR findings of primary ovarian granulosa cell tumor with focus on the differentiation with other ovarian sex cord-stromal tumorsJournal of Ovarian Research 2018 11(1):01-09.10.1186/s13048-018-0416-x29871662 [Google Scholar] [CrossRef] [PubMed]
[14]. Cheng H, Peng J, Yang Z, Zhang G, Prognostic significance of lymphadenectomy in malignant ovarian sex cord stromal tumor: A retrospective cohort study and meta-analysisGynecol Oncol 2018 148(1):91-96.10.1016/j.ygyno.2017.10.02229107349 [Google Scholar] [CrossRef] [PubMed]