Clear Cell Sarcoma of Kidney (CCSK) is a highly malignant renal tumour. The mean age of diagnosis is between 12-36 months. Due to heterogeneous histological appearance and age group affection similar to wilms tumour, it often leads to misdiagnosis. It has a tendency to metastasise distantly to bone and its therapeutic response differs from other childhood kidney tumours. So use of Immunohistochemical (IHC) markers becomes essential in many cases in differentiating CCSK from other paediatric renal neoplasms. It is extremely rare in adults, till now only 26 adult cases have been reported in the medical literature. Here, the case of a 49-year-old male presenting with haematuria and pedal oedema is reported. On radiological examination, he had a large left renal mass with tumour extension to Left Renal Vein (LRV) and Inferior Vena Cava (IVC) as thrombi. Histology and Immunohistochemistry (IHC) study revealed CCSK ruling the other differentials. This case is presented for its rarity in adult patients, unusual clinical feature of widespread vascular invasion rather than bone metastasis, simulating Renal Cell Carcinoma (RCC) and aggressive clinical behaviour. The diagnostic challenges faced by pathologist and clinicians further necessitate the proper diagnosis of the tumour for better management of such cases.
Fluid cytology, Haematuria, Renal vein, Vascular invasion
Case Report
A 49-year-old male presented with haematuria and Bilateral Lower (B/L) limb swelling since 15 days and acute retention of urine since four days. Recently, he was diagnosed with hypertension and had no other known co-morbidities. Physical examination revealed large mass present in the left side of abdomen and bilateral lower limb oedema. On routine investigations, the haematological and biochemical tests were unremarkable. Ascitic fluid cytology showed plenty of polymorphs, no dysplastic or malignant cells. He was extensively evaluated radiologically. Ultrasonography (USG) abdomen and pelvis revealed a well-defined heterogeneous Space Occupying Lesion (SOL) of size about 9×8.7×7.9 cm involving lower and mid pole of left kidney, likely neoplastic mass lesion. Contrast-Enhanced Computed Tomography (CECT) whole abdomen revealed a large hypodense mass lesion of size 9.7×10.1×9 cm occupying the entire kidney sparing a small part at the upper pole. It showed heterogeneous enhancement with internal necrosis [Table/Fig-1].
CECT shows a heterogeneous mass originating from the mid pole of the left kidney.
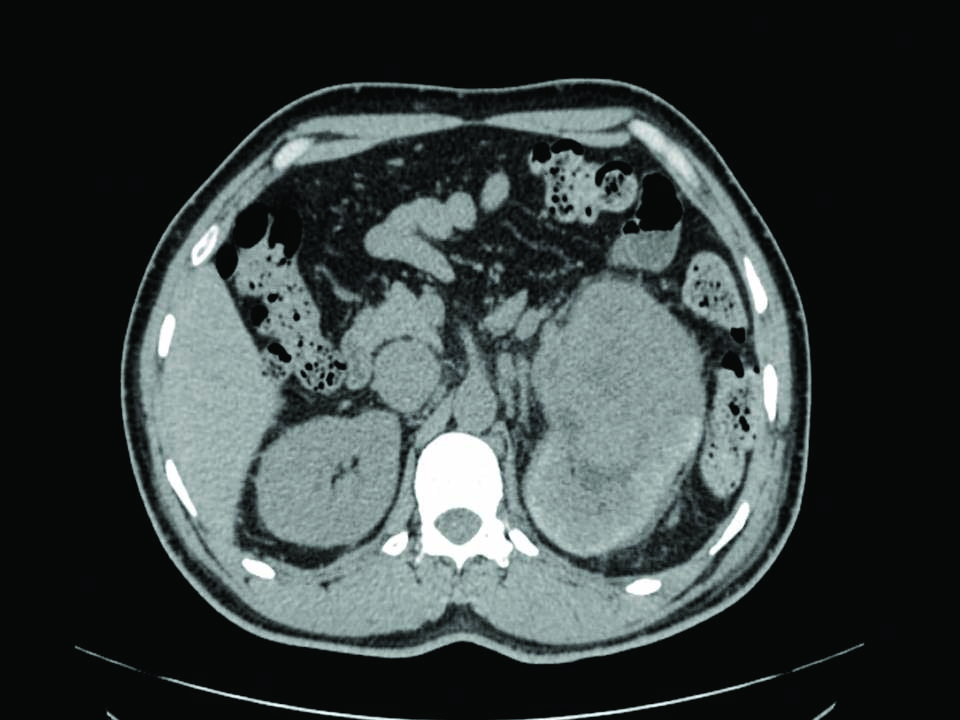
A tumour thrombus was invading entire LRV and adjacent IVC over a length of 4.0 cm extending upto B/L common iliac vein. Colour doppler study of B/L lower limb venous system revealed left common femoral vein, superficial femoral vein and popliteal vein having extensive intraluminal hypoechoic thrombus filling the lumen completely. However, posterior and anterior tibial veins appeared to be spared. CT thorax plain and bone scan showed no definitive scintigraphic evidence of metastasis. Hence, clinical diagnosis of left renal neoplasm with LRV and adjacent IVC invasion was made.
Patient underwent left radical nephrectomy with renal hilar and retrodudodenal lymph node dissection and IVC thrombus dissection. Intraoperative finding was a large left renal mass with dense adhesions to retroperitoneum and mesocolon infiltrating the psoas muscle. The tumour extended as thrombi to LRV, suprarenal part of IVC and bilateral common iliac veins. The radical nephrectomy specimen was sent to Department of Pathology for histopathological study.
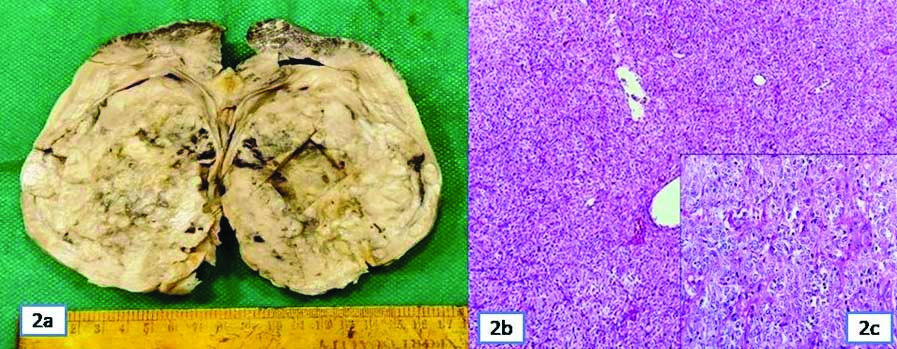
Grossly, it was a large well-circumscribed tumour in mid part of kidney involving the pelvic region and lower pole sparing upper pole. Cut section was greyish white variegated with areas of necrosis, cystic and mucoid change [Table/Fig-2]. Multiple sections studied in histopathology showed a tumour in renal parenchyma extending to renal pelvis, invading directly into renal vein and artery and ipsilateral adrenal gland. Tumour cells were monotonous and arranged in diffuse sheets, nested pattern and short fascicles. Cells were round to spindloid, had scanty pale cytoplasm, elongated to oval moderately pleomorphic vesicular nuclei and some showing conspicuous nucleoli. Focal clear cell change with areas of necrosis also noted. Mitosis was >19/10 High Power Field (HPF). The renal vein and ureteric cut margins, perinephric fat, Gerota’s fascia as well as renal hilar and retroperitoneal lymph nodes were free of tumour. Residual renal parenchyma revealed membroproliferative features in glomeruli and mild tubulointerstitial inflammation. Histological diagnosis was primary renal sarcoma. Differentials considered were RCC with sarcomatoid features, CCSK, rhabdoid tumour, Cellular Mesoblastic Nephroma (CMN) and synovial sarcoma. As all the differentials except RCC usually occurs in children and RCC also presents with vascular invasion, extensive regrossing was done. Still no areas of RCC could be identified in histomorphology. So the possibility of sarcomatoid change in RCC was ruled out first.
a) A well-circumscribed mass involving middle and lower pole of kidney; b) Tumour cells in diffuse sheets and nests (H&E ×40); c) Round to oval to spindloid cells with scanty cytoplasm (H&E ×100, inset).
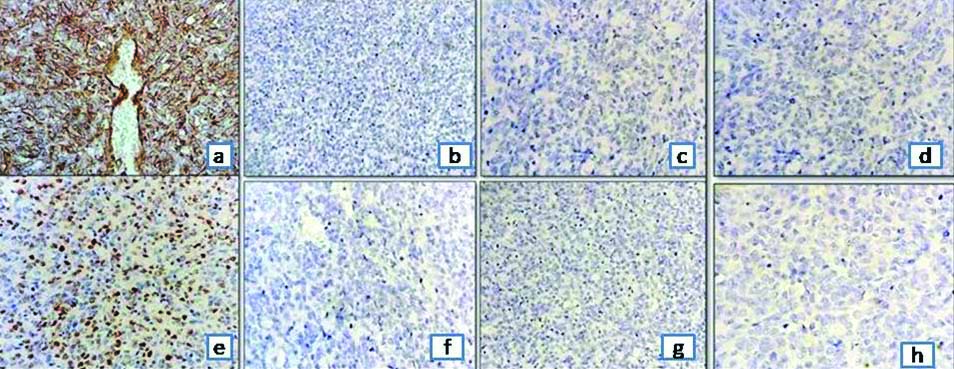
Further, IHC study of Paired Box Gene-8 (PAX8), Cytokeratin (CK), Epithelial Membrane Antigen (EMA), CD10, synaptophysin were negative, ruling out RCC, neuroendocrine tumour, mesoblastic nephroma and synovial sarcoma. Vimentin was strongly cytoplasmic positive in >90% of tumour cells and Cyclin D1 was strong nuclear positive in 50% of tumour cells [Table/Fig-3].
Immunostaining of the tumour cells: a) Vimentin positive (100x); b) Cytokeratin negative (100x); c) EMA negative (100x); d) WT1 negative (100x); e) Cyclin D1 focally positive (100x); f) CD99 negative (100x); g) Desmin negative (100x); and h) Synaptophysin negative (100x).
Though vimentin is also positive in rhabdoid tumour, the typical rhabdoid morphology was not evident in histology. Rhabdoid tumour also does not present with extensive intravascular invasion. So, final histological diagnosis was CCSK. As per American Joint Committee on Cancer (AJCC) guidelines, the pathologic stage was pT3 No MX and it was catagorised as stage II according to the updated National Wilms Tumour Study-5 (NWTS-5) definition [1,2].
Postoperative USG abdomen and pelvis showed non-visualisation of left kidney in left lumbar region with no intra-abdominal collections, but persistent thrombus in left external iliac vein. Postoperative period and hospital stay was uneventful.
Discussion
In 1978, CCSK was first recognised as an entity. Due to its tendency for metastasis to bone, it was named “bone metastasising renal tumour of childhood” [3]. It is an uncommon mesenchymal renal tumour constituting 3-4% of all primary childhood kidney tumours. Mean age of diagnosis is three years. Males are twice more affected than females in the ratio of 2:1 [4]. In adult patients till now, only 26 cases are reported [5]. The tumour is hypothesised to be originating from embryonic mesenchymal progenitor cells situated in medullary region of kidney [4,6].
Patients present with mass in abdomen, pain, haematuria along with systemic symptoms like fever, weight loss, nausea, vomiting and raised blood pressure [7]. Tumours are usually large in size, unilateral, unifocal, tan–grey coloured with areas of secondary changes like necrosis, cystic change and mucoid degeneration [8]. It is prone to invade renal venous system [6,9]. In this case, the tumour had extended into IVC and upto popliteal vein. In only 26 adult cases till reported, three cases had presence of IVC tumour thrombus and in two cases tumour thrombi had reached upto the right atrium [10,11]. Histomorphology shows varied architectural patterns like nests and cords separated by thin fibrovascular septae. Histologic variants are cellular, epitheloid, spindloid, anaplastic, myxoid, sclerosing and palisading [12]. CCSK closely mimicks other poorly differentiated renal tumours, like undifferentiated RCC, CMN, Malignant Rhabdoid Tumour of Kidney (MRTK), blastema-predominant wilms tumour, Primitive Neuroectodermal Tumour (PNET) and monophasic synovial sarcoma. A complete IHC panel including vimentin, CK, EMA, PAX 8, cyclin D1, WT-1, desmin, Smooth Muscle Actin (SMA), S100, synaptophysin are needed to rule out the differentials. RCC is positive for PAX 8, CK and vimentin. MRTK is focally positive for vimentin, S100, NSE (non-specific esterase) and synaptophysin. Cellular CMN shows cytoplasmic positivity for vimentin, desmin, and alpha-smooth muscle actin. PNET is positive for CD99, NSE, vimentin, S100, and synaptophysin.
Synovial sarcoma is EMA and CD 99 positive. WT1, desmin, NSE and CK are positive in blastema-predominant wilms tumour. Vimentin, cyclin D1 and Bcl-2 are typically reactive in CCSK and almost all other IHC markers are negative [13,14]. So, negativity of all these markers is more important than positivity for vimentin for diagnosing CCSK and excluding the differentials. Cytogenetic analysis has revealed chromosomal translocations at (1;6) (p32.3;q21) and gain of chromosome 1q and 11q in CCSK [15]. It is a highly aggressive recurring tumour with distant metastasis occuring to soft tissue, lung, liver and brain [16]. Different prognostic factors implicated are age at diagnosis, tumour necrosis, stage and treatment regimen. Prognosis has improved drastically with advent of newer diagnostic and treatment modalities. Radical nephrectomy followed by postoperative chemotherapy and radiation therapy is the treatment of choice now. Tumour responds very well to chemotherapy regimen comprising of doxorubicin, vincristine and actinomycin. With this the overall survival rate is now 69% [17].
Conclusion(s)
Clear Cell Sarcoma of Kidney (CCSK) in adults is uncommon. Varied and unusual morphology may cause delay in diagnosis. So, careful histological study and use of appropriate IHC markers is essential for its diagnosis. Ruling out the close, but prognostically different differential entities is of utmost importance as treatment modalities and response to therapy varies greatly among them.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA (Identity not revealed)
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Dec 30, 2020
Manual Googling: Feb 20, 2021
iThenticate Software: Mar 07, 2021 (10%)
[1]. Shao N, Wang HK, Zhu Y, Ye DW, Modification of American Joint Committee on cancer prognostic groups for renal cell carcinomaCancer Med 2018 7:5431-38.10.1002/cam4.179030306741 [Google Scholar] [CrossRef] [PubMed]
[2]. Bhatnagar S, Management of Wilms’ tumour: NWTS vs SIOPJ Indian Assoc Pediatr Surg 2009 14(1):06-14.10.4103/0971-9261.5481120177436 [Google Scholar] [CrossRef] [PubMed]
[3]. Amin MB, de Peralta-Venturina MN, Ro JY, El-Naggar A, Mackay B, Ordonez N, Clear cell sarcoma of kidney in an adolescent and in young adults: A report of four cases with ultrastructural, immunohistochemical, and DNA flow cytometric analysisAm J Surg Pathol 1999 23(12):1455-63.10.1097/00000478-199912000-0000210584698 [Google Scholar] [CrossRef] [PubMed]
[4]. Uddin N, Minhas K, Abdul-Ghafa J, Ahmed A, Ahmad Z, Expression of cyclin D1 in clear cell sarcoma of kidney. Is it useful in differentiating it from its histological mimics?Diagn Pathol 2019 14(1):1310.1186/s13000-019-0790-830736802 [Google Scholar] [CrossRef] [PubMed]
[5]. Kural AR, Onal B, Ozkara H, Cakarir C, Ayan I, Agaoglu FY, Adult clear cell sarcoma of the kidney: A case reportBMC Urol 2006 6:1110.1186/1471-2490-6-1116584570 [Google Scholar] [CrossRef] [PubMed]
[6]. Kattub H, Khalaf E, Maarraoui A, Mekresh ME, Adult clear cell sarcoma of the kidney: Review of literature and a case reportJCR 2014 4(1):159-63.10.17659/01.2014.0040 [Google Scholar] [CrossRef]
[7]. Benchekroun A, Zannoud M, el Alj HA, Nouini Y, Marzouk M, Faik M, Clear cell sarcoma of the kidney: 3 case reportsProg Urol 2002 12:469-73. [Google Scholar]
[8]. Balarezo FS, Joshi VV, Clear cell sarcoma of the pediatric kidney: Detailed description and analysis of variant histologic patterns of a tumour with many facesAdv Anat Pathol 2001 8:98-108.10.1097/00125480-200103000-0000611236959 [Google Scholar] [CrossRef] [PubMed]
[9]. Murphy WM, Beckwith JB, Farrow GM, Tumours of the kidney, bladder and related urinary structures. (Third Series, Fasicle 11) 1993 Washington DCThe Armed Forces Institute of Pathology:67-81. [Google Scholar]
[10]. Chen HT, Chen JT, Hung SW, Ou YC, Yang CR, Primary renal sarcoma with inferior vena cava thrombus presenting with tumour ruptureZhonghua Yi Xue Za Zhi (Taipei) 2001 64:183-86. [Google Scholar]
[11]. Rosso D, Ghignone GP, Bernardi D, Zitella A, Casetta G, De Zan A, Clear cell sarcoma of the kidney with invasion of the inferior vena cavaUrol Int 2003 70:251-52.10.1159/00006875612660471 [Google Scholar] [CrossRef] [PubMed]
[12]. Argani P, Perlman EJ, Breslow NE, Browning NG, Green DM, D’Angio GJ, Clear cell sarcoma of the kidney: A review of 351 cases from the National Wilms Tumour Study Group Pathology CenterAm J Surg Pathol 2000 24:04-18.10.1097/00000478-200001000-0000210632483 [Google Scholar] [CrossRef] [PubMed]
[13]. Wick MR, Ritter JH, Dehner LP, Malignant rhabdoid tumour: A clinicopathologic review and conceptual discussionSemin Diagn Pathol 1995 12:233-48. [Google Scholar]
[14]. Ellinger J, Bastian PJ, Hauser S, Biermann K, Müller SC, Primitive neuroectodermal tumour: Rare, highly aggressive differential diagnosis in urologic malignanciesUrology 2006 68:257-62.10.1016/j.urology.2006.02.03716904430 [Google Scholar] [CrossRef] [PubMed]
[15]. Sharma SC, Menon PA, Clear cell sarcoma of the kidneyJ Postgrad Med 2001 47:206-07. [Google Scholar]
[16]. Adanani A, Lalid R, Bouklata S, Ajana A, Hammani L, Imani F, Clear cell sarcoma of kidney in an adult: A case reportJ Radiol 2006 87:136-38.10.1016/S0221-0363(06)73985-3 [Google Scholar] [CrossRef]
[17]. Zhang Y, Jun Li J, Wang Y, Clear cell sarcoma of the kidney in a 62-year-old patient presenting with generalized pruritusBMC Cancer 2019 19(1034):02-09.10.1186/s12885-019-6212-131676003 [Google Scholar] [CrossRef] [PubMed]