Malignant Phyllodes Tumour with Rhabdomyosarcomatous Differentiation: A Rare Phenomenon
Kusum Yadav1, Jitendra Singh Nigam2, Anshul Singh3, Vatsala Misra4
1 Junior Resident (JR-3), Department of Pathology, Moti Lal Nehru Medical College, Prayagraj, Uttar Pradesh, India.
2 Assistant Professor, Department of Pathology/Lab Medicine, All India Institute of Medical Sciences, Patna, Bihar, India.
3 Associate Professor, Department of Pathology, Moti Lal Nehru Medical College, Prayagraj, Uttar Pradesh, India.
4 Professor and Head, Department of Pathology, Moti Lal Nehru Medical College, Prayagraj, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kusum Yadav, Junior Resident, Department of Pathology, Moti Lal Nehru Medical College, Lowther Road, George Town, Prayagraj, Uttar Pradesh, India.
E-mail: kusummedico9453@gmail.com
Phyllodes tumour is a rare tumour of the breast constituting less than 1% of all breast tumours. Malignant Phyllodes Tumour (MPT) accounts for only 10-30% of all phyllodes tumours. Heterologous sarcomatous differentiation in a MPT is an infrequent phenomenon, with the cases reported showing differentiation mostly towards liposarcoma, fibrosarcoma, angiosarcoma, osteosarcoma, or chondrosarcoma. MPTs with rhabdomyosarcomatous differentiation are scarcely seen with only three confirmed cases documented till date to the best of the knowledge after a thorough search of literature. Here, authors present a case of 45-year-old female who presented with a well-defined rapidly growing lump in the right breast for last one year. A core needle biopsy performed showed a sarcomatous picture on histology. Complete excision was subsequently done. On microscopy, most of the areas showed fibrosarcomatous changes with frequent mitoses. Some of the foci showed large pleomorphic cells in diffuse sheets that were polygonal with densely abundant eosinophilic cytoplasm and vesicular nucleus with prominent nucleoli (rhabdomyoblasts). Myogenin was diffusely positive on Immunohistochemistry (IHC). A diagnosis of MPT with rhabdomyosarcomatous differentiation was made. This case is reported here for its unusual presentation and to make pathologists aware of this rare heterologous differentiation of MPT.
Cystosarcoma phyllodes, Fibroepithelial tumours, Heterogeneity, Mammary sarcomas
Case Report
A 45-year-old female presented with the chief complaint of a well-defined, rapidly growing painless lump in the right breast for last one year. There was no significant past medical history. On examination, the lump was present in the right upper quadrant, well-defined, firm to hard, overlying skin was normal and there was no palpable axillary lymphadenopathy. Core needle biopsy was performed that showed a sarcomatous pathology [Table/Fig-1a]. A provisional differential diagnoses of metaplastic tumour and malignant mesenchymal tumour was thought of and a mammogram was advised. However, she didn’t get it done. A right-sided modified radical mastectomy was done. A well-encapsulated breast mass measuring 14×12×10 cm in size was received. Cut surface was multinodular and homogenous grey-white with few haemorrhagic areas [Table/Fig-1b]. Ten lymph nodes were dissected from the axillary tail.
a) Section from the initial biopsy showing a sarcomatous pathology (H&E, x10); b) Cut surface of the tumour showing homogenous grey-white areas; c) Section showing an intracanalicular growth pattern with leaf-like projections into dilated lumen (H&E x10); d) Section showing cellular stroma with maximum cellularity noted in zones closest to the compressed epithelial component appearing slit-like (*) (H&E x10); e) Section showing fibrosarcomatous changes in stroma with increased mitotic activity (H&E x10); f) Section showing diffuse sheets of polygonal cells with abundant densely eosinophilic cytoplasm and vesicular nucleus with prominent nucleoli (rhabdomyoblasts) (arrows) (H&E x40).
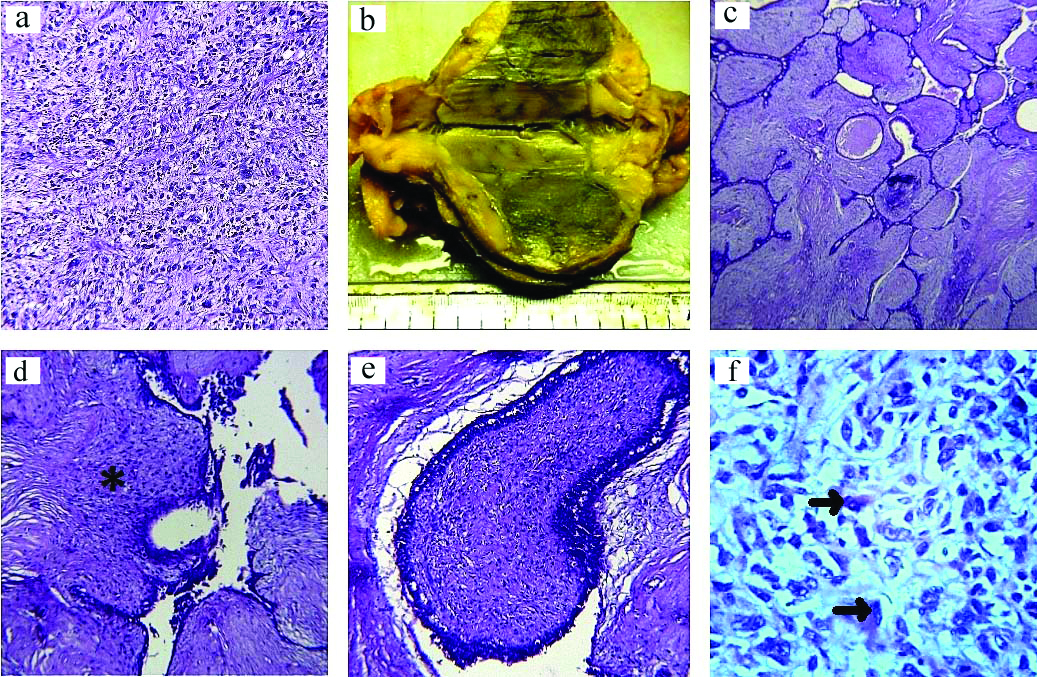
On microscopy, sections showed focal areas having an intracanalicular growth pattern with leaf-like projections into dilated lumen [Table/Fig-1c]. Rest of the areas showed a highly cellular stroma with marked overgrowth. The epithelial component was compressed into slit-like channels wherever appreciable, but was completely benign looking. Maximum cellularity of stroma was noted in zones closest to the epithelial component [Table/Fig-1d]. At many places stromal areas showed fibrosarcomatous changes with increased mitotic activity [Table/Fig-1e]. Occasional giant cells and areas of necrosis were also seen. In some of the foci, large pleomorphic cells were seen in diffuse sheets. These cells were polygonal with densely abundant eosinophilic cytoplasm and vesicular nucleus with prominent nucleoli [Table/Fig-1f].
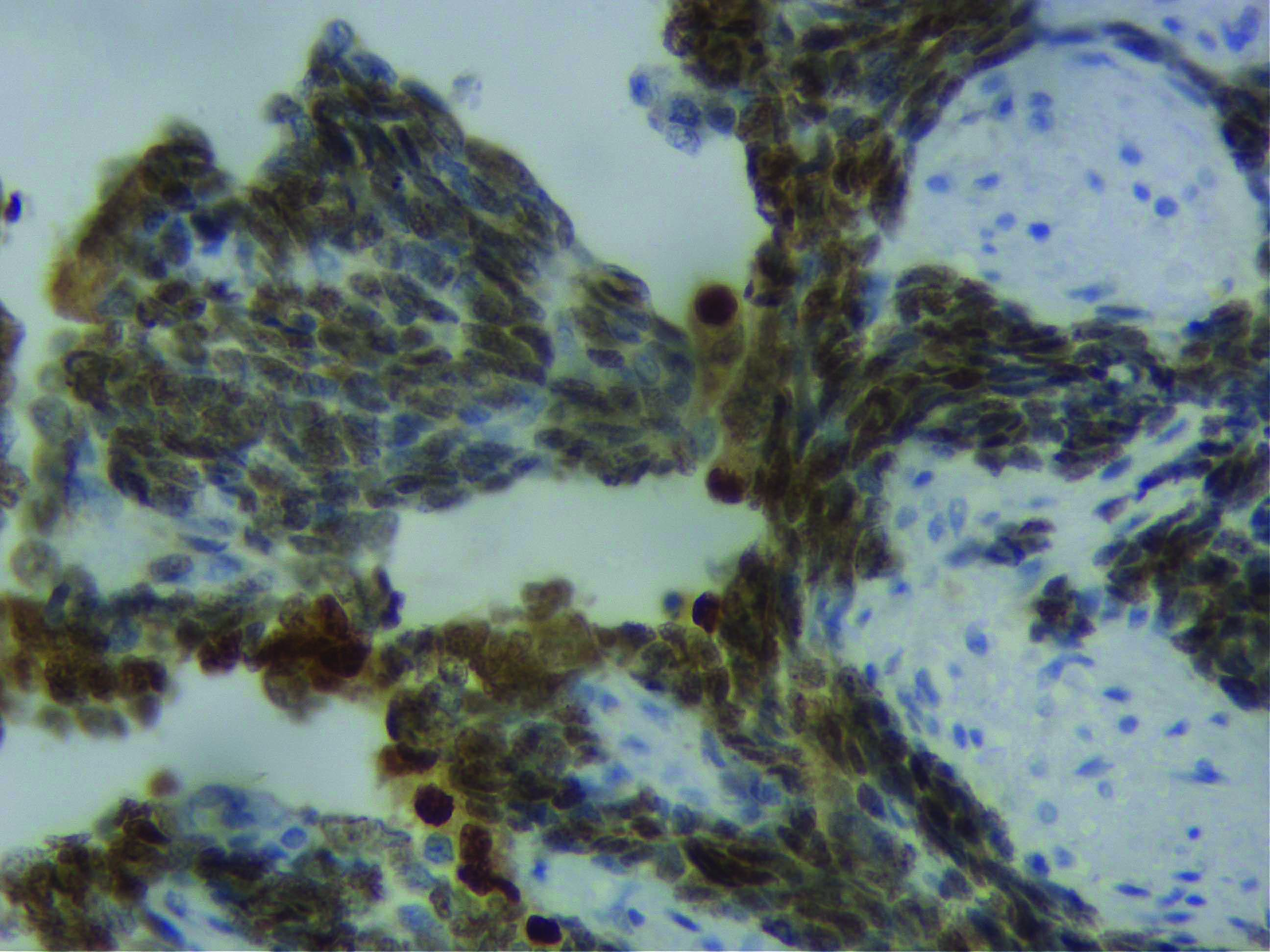
On the basis of these findings, a diagnosis of MPT was thought of. IHC for S100, desmin, CD34 and myogenin was applied to confirm the nature of these large pleomorphic cells, out of which myogenin turned out to be diffusely positive in these cells, confirming them to be rhabdomyoblasts [Table/Fig-2]. Hence, a diagnosis of MPT with rhabdomyosarcomatous differentiation was finally rendered. Following this the patient was given adjuvant chemotherapy and she was doing fine with no evidence of any recurrence/metastasis in the follow-up period of six months.
Cells diffusely positive for myogenin (IHC X400).
Discussion
Phyllodes tumour is a rare breast tumour which accounts for less than 1% of all breast neoplasms and 2.5% of all fibroepithelial breast lesions [1]. In the women of Asian origin, however, the incidence of phyllodes is higher (6.92%) [2]. Although most phyllodes tumours occur in women between the ages of 35-55 years, adolescent and elderly women can also be affected [3].
Phyllodes tumours form a spectrum from absolutely benign to borderline to frankly malignant tumours based on a constellation of histological characteristics that comprise the degree of stromal overgrowth, hypercellularity, cytologic atypia, mitotic activity and circumscribed vs. invasive margins [3]. Majority of these lesions fall in the benign and borderline categories that constitute approximately 70-90% of the cases. MPTs are very rare forming only 10-30% of all phyllodes tumours [4]. They are very aggressive with a 25% chance of distant metastasis. They show a wide variation in the spectrum of histological appearances with both completely undifferentiated sarcomas as well as sarcomas showing heterologous differentiation being reported [1,2].
Clinically, MPTs appear as a round, mobile and painless mass with the tumour size ranging from 1-40 cm [4]. In most patients, axillary lymph nodes are not palpable at presentation, as metastasis to these occurs in only 2%. Metastatic spread of these tumours is primarily haematogenous with lungs, pleura and bone being the most common sites [1,2].
Benign to borderline phyllodes tumour, grossly show leaf-like fronds projecting into cystic spaces that on microscopic examination consist of epithelial-lined stromal projections protruding into dilated/cystic spaces [5,6]. However, most MPTs are solid and vaguely lobulated with the well developed leaf-like fronds being conspicuously absent. On histopathology, marked fibrosarcomatous changes of the stromal component are seen with the epithelial component being absent/negligible [1,7]. A thorough search of literature shows that heterologous sarcomatous transformation towards liposarcoma, fibrosarcoma, angiosarcoma, osteosarcoma, chondrosarcoma, osteoclast like giant cells and rhabdomyosarcoma have been occasionally encountered in the said order of frequency [1,2,4-6,8-11]. This has diagnostic significance as finding of a malignant heterologous element places the tumour straightaway into a malignant category, even in the absence of specific criteria required to fulfil the diagnosis of a malignant transformation in a phyllodes tumour [10]. However, recent literature suggests that liposarcoma as a heterologous component is no longer a histological criteria of malignancy, because atypical adipocytes in liposarcoma do not harbour MDM2 aberrations or CDK4 amplification [3].
As far as Rhabdomyosarcomas (RMS) occurring in breast is concerned, they are also exceedingly rare. Mostly present as pure neoplasms, metastasis from other sites being more common than a primary. They affect exclusively children and adolescent females; though rare cases in middle-aged females have been documented. The most common histologic subtype is alveolar, irrespective of whether primary or metastatic. These tumours are usually treated by the combined approach of surgery with radiotherapy/chemotherapy. They have an overall dismal prognosis, with the metastatic ones carrying an extremely poor prognosis [12,13].
The closest differential of pure RMS of breast is rhabdomyosarcomatous MPT, especially in those cases showing a pleomorphic subtype of histology. Till date only three cases of confirmed rhabdomyosarcomatous differentiation in MPTs have been published, making this case as the fourth one [9-11]. All of these were reported in middle-aged females which was similar to the age of present case. In two cases, the tumour was entirely solid, similar to present one, whereas in one it was solid cystic. All of them showed frequent rhabdomyoblasts with prominent cross-striations. However, in present case, the cross-striations were not evident on light microscopy, so the help of IHC for confirmation was taken. Osteoclast like giant cells was seen in one case, which was also present in this case. None of these cases showed axillary node involvement, which was absent in present case too [Table/Fig-3].
Published literature on malignant phyllodes with rhabdomyosarcomatous differentiation [9-11].
| Author | Year | Salient features |
|---|
| Age (years)/Sex | Consi-stency | Rhabdomyoblast | Other cells | Necrosis | Lymph node | Follow-up period | Epithelial components |
|---|
| Barnes L and Pietruszka M, [9] | 1978 | 45/Female | Solid | Frequent and prominent cross-striations on light microscopy. No IHC done | - | Large areas | 14 lymph nodes, all free of tumour | 2.5 years, metastasis to lung and brain, succumbed | Negligible |
| Tan PH et al., [10] | 2005 | Middle age/Female | Solid | Frequent and prominent cross-striations on light microscopy. No IHC done | - | Present | No lymph nodes dissected | Not mentioned | Epithelial component was compressed into slit-like channels. |
| Diwan R et al., [11] | 2012 | 40/Female | Solid cystic | Frequent and prominent cross-striations on light microscopy. No IHC done | Few Osteoclast like giant cells | Present | No lymph nodes dissected | Not mentioned | Epithelial component was compressed into slit-like channels. |
| Yadav K et al., (present case) | 2020 | 45/Female | Solid | Few cross-striations not evident. On light microscopy. IHC for S100, desmin, CD34 and myogenin was done, out of which myogenin was diffusely positive. | Occasional osteoclast like giant cells | Spotty necrosis | 10 lymph nodes, all free of tumour | Six months, uneventful | Epithelial component was compressed into slit-like channels. |
Genetic abnormalities associated with phyllodes tumours are loss of nuclear Beta-Catenin, amplification of MYC and aberrant expression of TP53. Other described cytogenetic changes seen specifically in the malignant ones include gain in chromosome 1q and losses at chromosome 13 [3,14].
The prognosis of benign and borderline phyllodes tumour is quite good with the overall survival rate of 91% at five years. The only issue of concern in these tumours is recurrence which is around 15%. But the five-year survival rate for MPTs is around 80% only [3]. Treatment of MPTs is complete surgical excision with wide clear margins of at least 1 cm. Unlike primary RMS, where adjuvant radiation therapy/chemotherapy are beneficial, their role in MPTs with rhabdomyosarcomatous differentiation is not very well known because of the rarity of such cases. MPTs with distant metastasis show rapid progression and adjuvant therapies in these cases if used may rarely be effective leading to high mortality [14].
Concusion(s)
Malignant Phyllodes Tumour (MPT) with rhabdomyosarcomatous differentiation is an extremely rare phenomenon. This case was considered worth reporting to make pathologists aware of the entity, the necessity of differentiation with pure mammary RMS and its clinical implications to avoid potential diagnostic pitfalls and patient mismanagement.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 05, 2020
Manual Googling: Jan 22, 2021
iThenticate Software: Mar 09, 2021 (11%)
[1]. Nayak M, Patra S, Mishra P, Sahoo N, Sasmal PK, Mishra TS, Malignant phyllodes tumour with heterologous differentiation: Clinicopathological spectrum of nine cases in a tertiary care institute in Eastern IndiaIndian J Pathol Microbiol 2017 60:371-76.10.4103/IJPM.IJPM_426_1628937374 [Google Scholar] [CrossRef] [PubMed]
[2]. Okaly GVP, Devadass CW, Metikurke SH, Malignant phyllodes tumour with heterologous differentiation: A rare case reportJ Can Res Ther 2015 11:651-55.10.4103/0973-1482.13799626458626 [Google Scholar] [CrossRef] [PubMed]
[3]. Tse G, Koo JS, Thike AA, Phyllodes tumour In: Lokuhetty D, White VA, Watanabe R, Cree IA (eds.)WHO Classification of Tumours of the Breast 2019 2Lyon (France)IARC:172-76. [Google Scholar]
[4]. Pornchai S, Chirappapha P, Pipatsakulroj W, Lertsithichai P, Vassanasiri W, Sitathanee C, Malignant transformation of phyllodes tumour: A case report and review of literatureClin Case Rep 2018 6:678-85.10.1002/ccr3.142829636939 [Google Scholar] [CrossRef] [PubMed]
[5]. Kraemer B, Hoffman J, Roehm C, Gall C, Wallweiner P, Krainick-Strobel U, Cystosarcoma Phyllodes of the breast: A rare diagnosis. Case studies and review of literatureArch Gynecol Obstet 2007 276:649-53.10.1007/s00404-007-0393-617549503 [Google Scholar] [CrossRef] [PubMed]
[6]. Esposito NN, Mohan D, Brufsky A, Lin Y, Kapali M, Dabbs DJ, Phyllodes tumour: A clinicopathologic and immunohistochemical study of 30 casesArch Pathol Lab Med 2006 130:1516-21.10.5858/2006-130-1516-PTACAI17090194 [Google Scholar] [CrossRef] [PubMed]
[7]. Rosen PP, Fibroepithelial neoplasms. In: Weinberg RW, Donnellan K, Palumbo R (ed.)Rosen’s Breast Pathology 2001 2nd edPhiladelphiaLippincott Williams & Wilkins:176-200. [Google Scholar]
[8]. Vani BR, Kumar BD, Sandhyalakshmi BN, Geethamala K, Murthy VS, Radha M, Malignant phyllodes tumour with osteoclastic giant cellsSaudi J Health Sci 2014 3:124-26.10.4103/2278-0521.134868 [Google Scholar] [CrossRef]
[9]. Barnes L, Pietruszka M, Rhabdomyosarcoma arising within a cystosarcoma phyllodes. Case report and review of the literatureAm J Surg Pathol 1978 2:423-29.10.1097/00000478-197812000-00009216277 [Google Scholar] [CrossRef] [PubMed]
[10]. Tan PH, Jayabaskar T, Chuan KL, Lee HY, Tan Y, Hilmy M, Phyllodes tumours of the breast: The role of pathologic parametersAm J Clin Pathol 2005 123:529-40.10.1309/U6DVBFM81MLJC1FN15743740 [Google Scholar] [CrossRef] [PubMed]
[11]. Diwan R, Mathur M, Mathur D, Malignant phyllodes tumour with giant cells and rhabdomyosarcomatous differentiation: A case reportInt J Med Sci Public Health 2012 1:147-49.10.5455/ijmsph.2012.147-149 [Google Scholar] [CrossRef]
[12]. Pareekutty NM, Bhagat M, Vora T, Qureshi SS, Rhabdomyosarcoma of the breast: Report of two cases with the review of literatureJ Indian Assoc Pediatr Surg 2016 21:81-83.10.4103/0971-9261.17696427046981 [Google Scholar] [CrossRef] [PubMed]
[13]. Tan PH, Tse G, Lee A, Simpson JF, Hanby AM, Fibroepithelial tumours In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ (eds.)WHO Classification of Tumours of the Breast 2012 LyonIARC:142-47. [Google Scholar]
[14]. Kim JY, Yu JH, Nam SJ, Kim SW, Lee SK, Park WY, Genetic and clinical characteristics of phyllodes tumours of the breastTransl Oncol 2018 11:18-23.10.1016/j.tranon.2017.10.00229145046 [Google Scholar] [CrossRef] [PubMed]