Carcinosarcoma Stomach with Biphasic Nodal Metastasis: A Rare Entity
Renu Sukumaran1, Jayasree Katoor2, Arun Peter Mathew3
1 Associate Professor, Department of Pathology, Regional Cancer Centre, Thiruvananthapuram, Kerala, India.
2 Professor, Department of Pathology, Regional Cancer Centre, Thiruvananthapuram, Kerala, India.
3 Additional Professor, Department of Surgical Oncology, Regional Cancer Centre, Thiruvananthapuram, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Renu Sukumaran, Associate Professor, Division of Pathology, Regional Cancer Centre, Thiruvananthapuram, Kerala, India.
E-mail: renu.sukumaran@gmail.com
Gastric carcinosarcoma is an extremely rare, aggressive, biphasic tumour composed of a mixture of carcinomatous and sarcomatous elements. Clinical symptoms and imaging studies of carcinosarcoma are not different from that of carcinoma. Biopsy of the lesions may not include both components. Thus, a diagnosis of carcinosarcoma is often rendered in surgical specimens. Immunohistochemistry will help to identify the various components. The relative proportion of the carcinomatous and sarcomatous elements is variable. One of the components may dominate the histologic picture. The common carcinomatous component described is adenocarcinoma with rare cases, showing neuroendocrine and squamous elements. The sarcomatous elements include spindle cell sarcoma which was Not Otherwise Specified (NOS), leiomyosarcoma, fibrosarcoma, osteosarcoma, chondrosarcoma, liposarcoma, undifferentiated sarcoma, myxoid sarcomas, and rhabdomyosarcoma. Herein, the authors are reporting the case of a 62-year-old female patient who presented with abdominal pain and vomiting of three-month duration. Endoscopic examination revealed a large polypoidal mass lesion in the body of stomach. Subtotal gastrectomy was done which showed large polypoidal mass lesion measuring 12×9.5×6 cm. Histopathological examination revealed a biphasic neoplasm with close intermingling of malignant epithelial and mesenchymal elements. Carcinoma component showed glandular, squamous and neuroendocrine areas. The sarcomatous component was spindle cell sarcoma, NOS. On immunohistochemical examination, epithelial component showed cytokeratin positivity. The neuroendocrine component was positive for synaptophysin, chromogranin A and CD56. The p40 positivity noted in the squamous component. The mesenchymal component showed positivity for vimentin. The nodal metastasis showed admixture of carcinoma (glandular and squamous components) and sarcoma.
Neuroendocrine component, Squamous cell carcinoma, Stomach carcinosarcoma
Case Report
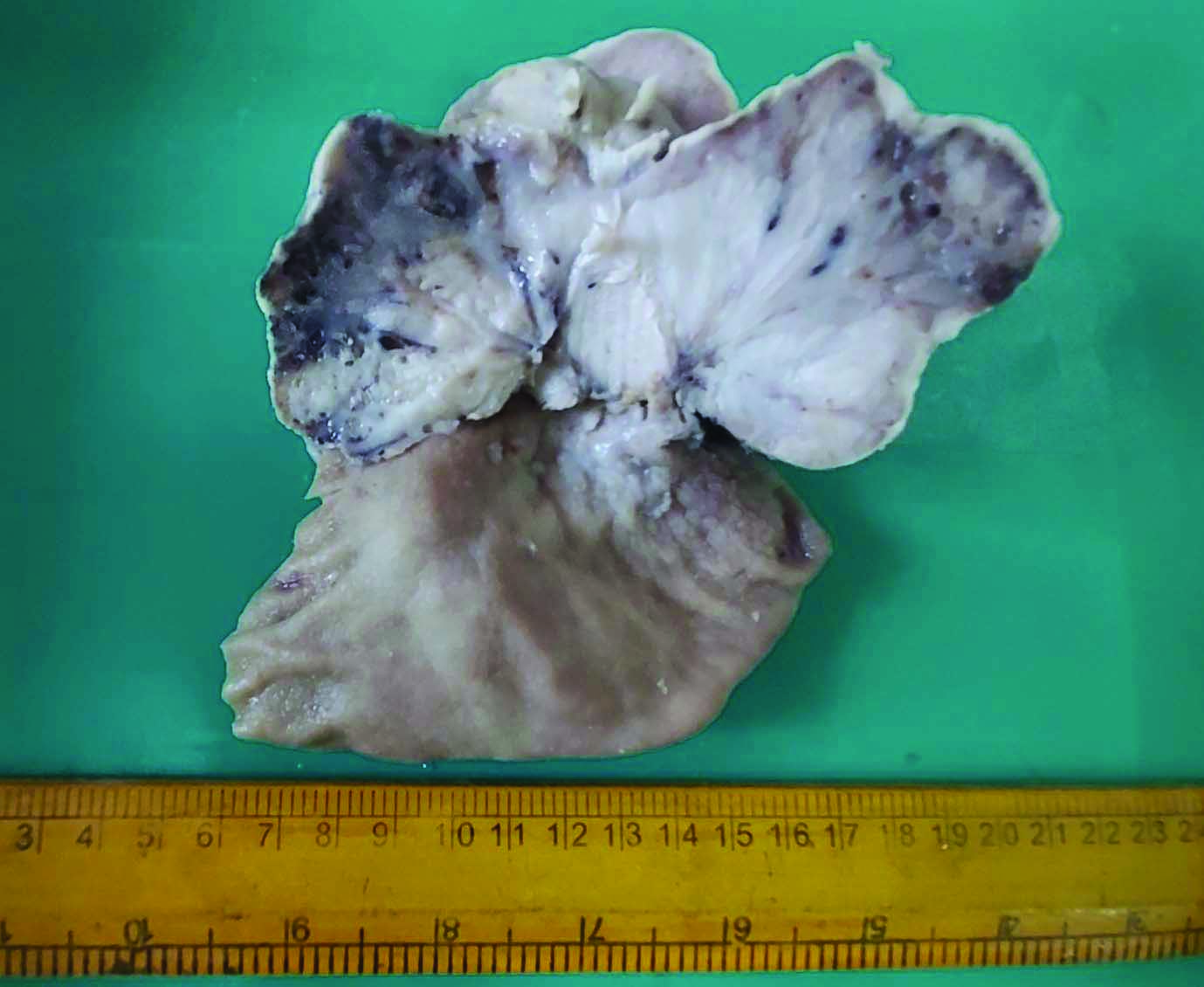
A 62-year-old female patient presented with abdominal pain, epigastric discomfort and vomiting of three-month duration. There was no significant past medical history. Endoscopic examination revealed a large polypoidal mass lesion in the posterior wall of body of stomach. Computerised tomography scan showed proliferative lesion involving antrum and anterior wall of stomach. There were enlarged perigastric nodes. No evidence of distant metastasis or infiltration to adjacent structures seen. With a clinical diagnosis of carcinoma stomach, biopsy of the lesion was taken which revealed atypical epithelial and stromal components. Subtotal gastrectomy with Roux- en- Y gastrojejunostomy was done which showed large polypoidal mass lesion measuring 12×9.5×6 cm [Table/Fig-1]. Cut surface was grey white with areas of haemorrhage. Histopathological examination revealed a biphasic neoplasm with close intermingling of malignant epithelial and mesenchymal elements.
Subtotal gastrectomy specimen with large polypoidal mass.
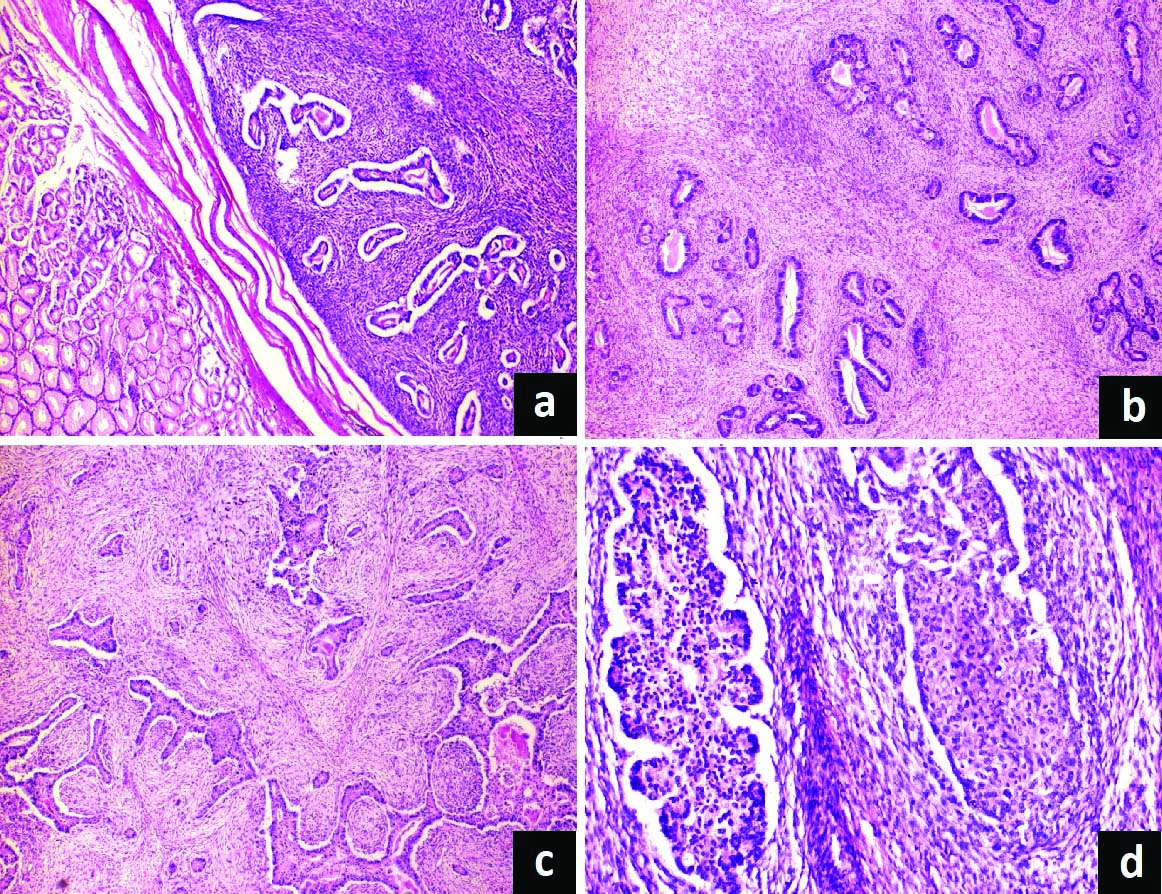
The epithelial component consisted of irregular glands lined by cuboidal to columnar epithelium, vesicular nuclei with anisonucleosis, some showing prominent nucleoli. Foci with squamous differentiation noted. In areas, cells with granular chromatin were arranged in trabeculae and rosettoid pattern [Table/Fig-2].
a) Biphasic neoplasm with close intermingling of malignant epithelial and mesenchymal elements (H&E, X50); b) glandular and spindle cell components (H&E, X50); c) neuroendocrine and spindle cell components (H&E,X50); d) squamous, neuroendocrine and spindle cell components (H&E,X100).
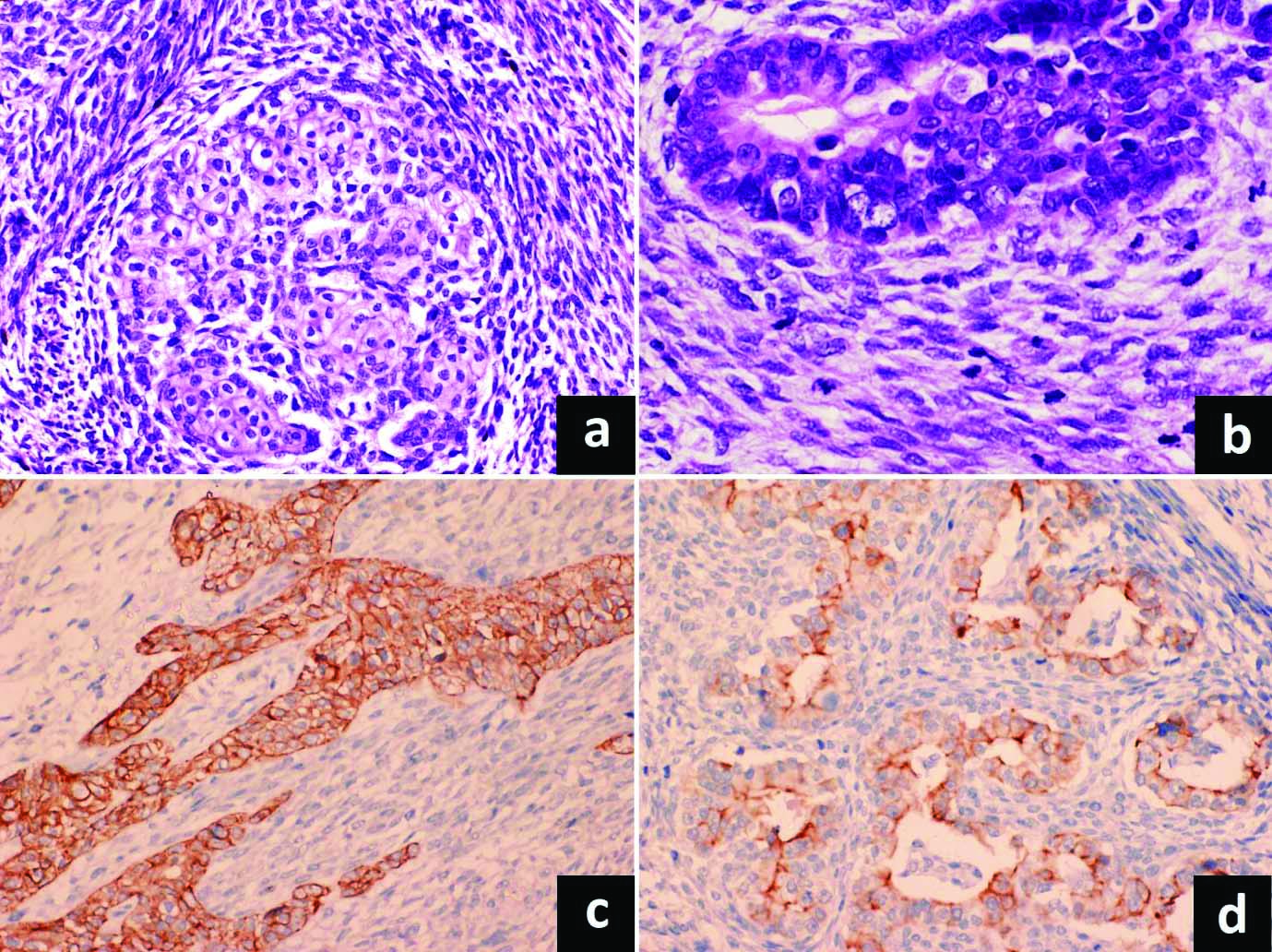
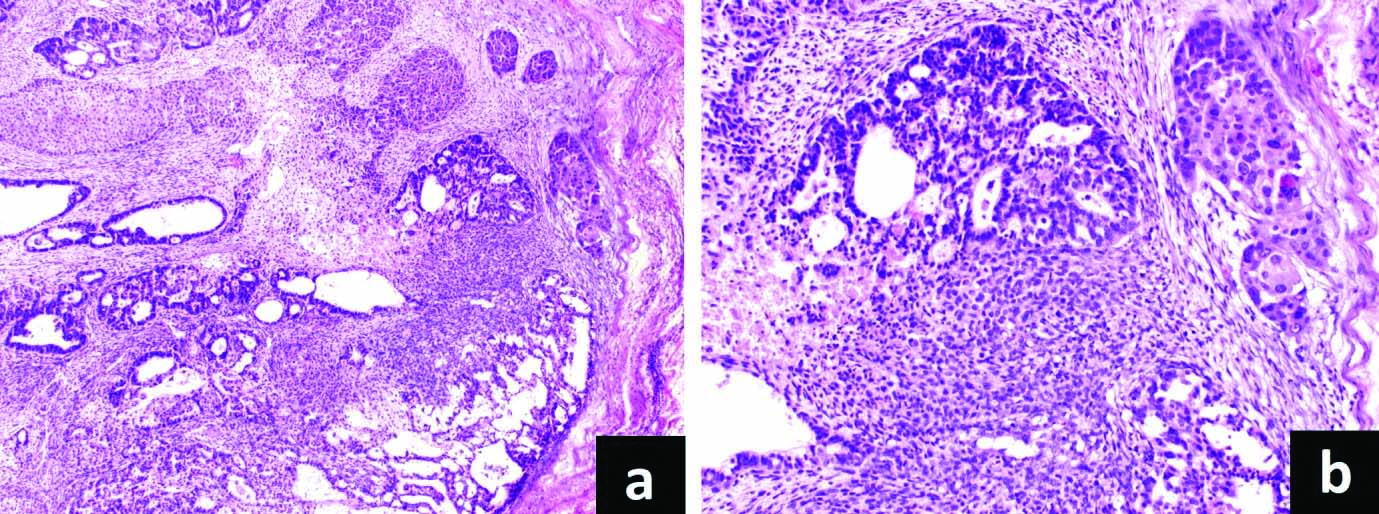
Close intermingling of mesenchymal component noted, consisting of fascicles of atypical spindly cells with plump ovoid atypical nuclei. Brisk mitotic activity including atypical mitoses noted both in the epithelial and mesenchymal components [Table/Fig-3]. The tumour cells were infiltrating the submucosa of the stomach. Of the 16 lymph nodes, two nodes were showing metastasis. The nodal metastasis showed admixture of carcinoma (glandular and squamous components) and sarcoma [Table/Fig-4a,b]. Diagnosis of carcinosarcoma stomach with biphasic nodal metastasis was given.
a) Higher power view showing malignant spindle cell and squamous areas (H&E, X200); b) brisk mitotic activity in spindle cell and glandular areas (H&E, X400); c) Synaptophysin positivity in neuroendocrine component (IHC, X200); d) Chromogranin positivity (IHC, X200).
a) Nodal metastasis showing admixture of carcinoma (glandular and squamous components) and sarcoma (H&E, X50); b) higher power highlighting the different components (H&E, X200).
On immunohistochemical examination, epithelial component showed cytokeratin positivity. The neuroendocrine component was positive for synaptophysin, chromogranin A and CD56 [Table/Fig-3c,d]. The p40 positivity noted in the squamous component. The mesenchymal component showed positivity for vimentin whereas the lineage-specific immunohistochemical markers- S100, SMA, desmin and CD117 were negative. Final diagnosis of carcinosarcoma stomach with carcinoma component showing glandular, squamous and neuroendocrine areas, and sarcomatous component of spindle cell sarcoma, NOS was given.
Discussion
Carcinosarcoma is a rare entity characterised by the presence of malignant epithelial and mesenchymal components. Aetiology and pathogenesis of this tumour is still unclear [1]. Uterus is the most common site of carcinosarcoma. Other less common sites described include ovary, breast, skin, salivary gland, lung, thyroid gland, urinary tract and prostate [2]. Carcinosarcoma represent approximately 3% of all oesophageal tumours, but gastric localisation is extremely rare [3]. Identification of the co-existence of carcinomatous and sarcomatous components on microscopic examination and the subclassification of different components based on immunohistochemical studies is critical for the diagnosis. Metastases may contain either sarcoma, carcinoma, or both [4].
Literature search show only limited number of cases of carcinosarcoma of stomach. The first case of carcinosarcoma of the stomach was described by Queckenstedt in 1904. These tumours exhibit aggressive behaviour [5]. Carcinosarcomas of the stomach may present as polypoidal, exophytic or endophytic lesions. These tumours are generally rapidly growing, large friable lesions with a variegated appearance showing areas of haemorrhage and necrosis. Diagnostic histopathological feature is the presence of both carcinomatous and sarcomatous components. Diagnosis can be challenging when any of these components dominate. The most common carcinoma component is adenocarcinoma. Rare cases with neuroendocrine differentiation are also described [2,6,7]. There are isolated case reports which demonstrate squamous differentiation [8]. The sarcomatous components include spindle cell sarcoma NOS, leiomyosarcoma, myxoid sarcomas, fibrosarcoma, osteosarcoma, chondrosarcoma, liposarcoma, undifferentiated sarcoma and rhabdomyosarcoma [3,6-8].
Spindle cell (sarcomatoid) carcinoma is the main differential diagnosis of carcinosarcoma. In sarcomatoid carcinoma, the spindle cell/sarcomatoid components have either immunohistochemical or ultrastructural features of epithelial differentiation. In carcinosarcoma, the sarcomatous components correspond to a well-defined type of soft tissue sarcoma. Carcinoma with benign bony or cartilaginous metaplasia should be differentiated from carcinosarcoma. In carcinosarcoma, the malignant nature of both epithelial and the mesenchymal components must be clearly recognised on microscopic examination.
The relative proportion of the epithelial and mesenchymal components is variable in carcinosarcoma. When dealing with primary malignant mesenchymal neoplasms of the stomach, the possibility of mesenchymal predominant carcinosarcoma should always be considered in the differential diagnosis. Extensive sampling and meticulous search for any epithelial malignant component is mandatory. Differential diagnoses also include a wide variety of malignant tumours such as gastrointestinal stromal tumour, leiomyosarcoma, malignant peripheral nerve sheath tumour with a heterologous component and biphasic synovial sarcoma [1,5]. Histopathogenesis of carcinosarcoma still remains controversial and unknown. Two theories exist regarding the pathogenesis [1,3,5]. The convergence hypothesis (biclonal origin hypothesis) states that carcinosarcoma originate from two different tumour cell clones i.e., both tumours have different cells of origin. Tumour with a distinct boundary between the two components represent collision tumour. When there is intermingling between the two components, it is called a composite tumour.
According to the divergence hypothesis (monoclonal origin hypothesis), a common pluripotential stem cell that is capable of undergoing divergent differentiation give rise to both the epithelial and mesenchymal components of the tumour. Some researchers postulate that the epithelial cells of carcinoma undergoing a metaplastic sarcomatous transformation represents sarcomatous component of the tumour [1,3,5,8]. Immunohistochemical, ultrastructural, and molecular genetic studies suggest and favour monoclonal origin hypothesis. Studies have found identical mutations of P53 and KRAS in both epithelial and mesenchymal components of carcinosarcoma, supporting the monoclonal origin hypothesis of carcinosarcoma. Literature search show the age range of patients affected by gastric carcinosarcoma is from 29 to 80 years and the median age is 62 years. The median dimension of the tumour is 9 cm, ranging from 4 cm to 15 cm [1,5].
Metastases from carcinosarcomas most often show pure carcinoma component. Rare cases with metastatic foci showing both carcinomatous and sarcomatous components have been described. The occurrence of pure sarcoma in the metastatic focus is exceptionally rare [3,4]. Carcinosarcomas are aggressive malignancies with a generally poor prognosis [5]. Current scientific evidence suggests the most important prognostic factor is the stage of tumour at the time of diagnosis. Radical gastrectomy is the treatment of choice. Even after curative treatment, the mean survival period is estimated to be about 10-15 months [2]. Overall tumour recurrence of this type of tumour in the first postoperative year is greater than 50% [2,8].
Conclusion(s)
Gastric carcinosarcoma is a rare biphasic tumour composed of mixture of malignant epithelial and mesenchymal elements. Proportion of the two components may vary. When any of these components dominate, the diagnosis can be challenging. Accurate categorisation is important as these tumours are aggressive with poor prognosis.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 01, 2020
Manual Googling: Jan 19, 2021
iThenticate Software: Feb 17, 2021 (9%)
[1]. Cirocchi R, Trastulli S, Desiderio J, Grassi V, Barillaro I, Santoro A, Gastric carcinosarcoma: A case report and review of the literatureOncol Lett 2012 4:53-57.10.3892/ol.2012.69922807959 [Google Scholar] [CrossRef] [PubMed]
[2]. Teramachi K, Kanomata N, Hasebe T, Ishii G, Sugito M, Ochiai A, Carcinosarcoma (pure endocrine cell carcinoma with sarcoma components) of the stomachPathol Int 2003 53:552-56.10.1046/j.1440-1827.2003.01508.x12895235 [Google Scholar] [CrossRef] [PubMed]
[3]. Randjelovic T, Filipovic B, Babic D, Cemerikic V, Filipovic B, Carcinosarcoma of the stomach: A case report and review of the literatureWorld J Gastroenterol 2007 13:5533-36.10.3748/wjg.v13.i41.553317907304 [Google Scholar] [CrossRef] [PubMed]
[4]. Kayaselcuk F, Tuncer I, Toyganözü Y, Bal N, Ozgür G, Carcinosarcoma of the stomachPathol Oncol Res 2002 8:275-77.10.1007/BF0303674512579216 [Google Scholar] [CrossRef] [PubMed]
[5]. Di Marco F, Piombino E, Portale TR, Magro G, Pesce A, Carcinosarcoma of the stomach: A rare tumor for an unusual localization. Review of the literatureTurk J Gastroenterol 2019 30:1066-69.10.5152/tjg.2019.1907731854314 [Google Scholar] [CrossRef] [PubMed]
[6]. Jang SM, Jang SH, Min KW, Na W, Jun YJ, Paik SS, A case of gastric carcinosarcoma with neuroendocrine and smooth muscle differentiationKorean J Pathol 2010 44:87-91.10.4132/KoreanJPathol.2010.44.1.87 [Google Scholar] [CrossRef]
[7]. Yamazaki K, A gastric carcinosarcoma with neuroendocrine cell differentiation and undifferentiated spindle-shaped sarcoma component possibly progressing from the conventional tubular adenocarcinoma; An immunohisto-chemical and ultrastructural studyVirchows Arch 2003 442:77-81.10.1007/s00428-002-0725-712536318 [Google Scholar] [CrossRef] [PubMed]
[8]. Sato Y, Shimozono T, Kawano S, Toyoda K, Onoe K, Asada Y, Hayashi T, Gastric carcinosarcoma, coexistence of adenosquamous carcinoma and rhabdomyosarcoma: A case reportHistopathology 2001 39:543-44.10.1046/j.1365-2559.2001.1301e.x11737318 [Google Scholar] [CrossRef] [PubMed]