Foetal Nasal Bone Length Measurement and Evolving Normogram in Southern Indian Population in Normal Pregnancies: A Cross-sectional Study
RB Prakash Jain1, Sanket M Kotnis2, HN Roopa3
1 Associate Professor, Department of Radiology, Sri Siddhartha Institute of Medical Sciences and Research Centre, Bangalore, Karnataka, India.
2 Assistant Professor, Department of Radiology, Sri Siddhartha Institute of Medical Sciences and Research Centre, Bangalore, Karnataka, India.
3 Assistant Professor, Department of Radiology, Rajarajeshwari Medical College and Research Centre, Bangalore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: RB Prakash Jain, Associate Professor, Department of Radiology, Sri Siddhartha Institute of Medical Sciences and Research Centre, Bangalore, Karnataka, India.
E-mail: doctorpjain@gmail.com
Introduction
Assessment of the foetus to detect aneuploidies between 12 to 24 weeks of gestation is an integral part of the ultrasound scanning. Absence or hypoplastic nasal bone is a very important marker of foetal aneuploidy. Thus, identifying the range of foetal Nasal Bone Length (NBL) in normal gestation is very important to screen for aneuploidies.
Aim
To ascertain the reference range of foetal NBL between 11 to 24 weeks of pregnancy.
Materials and Methods
A multicentre, cross-sectional study was conducted from November 2018 to April 2020. After obtaining Ethical approvals from each centre, NBL was measured in 826 patients between 11-24 weeks of pregnancy. Transabdominal scans were performed and the data was used to construct the NBL normogram for this Gestational Age (GA). Linear regression analysis was used to analyse the relationship between NBL and Gestational Age. Scatter plots for NBL as function of GA was constructed. The 5th and 50th percentile values were calculated for each gestational week.
Results
With one unit increase in GA (weeks), NBL increased by 0.402 times (r=0.897, p<0.001). Mean NBL was 4.05 mm. The medians NBL for 12-14 weeks were 2.1-2.5 mm, 15-18 weeks was 3.0-4.6 mm, 19-21 weeks was 5.3-5.8 mm, 22-24 weeks was 6.0-6.6 mm.
Conclusion
This study highlights the importance of nasal bone evaluation in the second trimester of pregnancy to detect foetus with Down syndrome. Since NBL increases linearly with GA, it aids detecting the hypoplastic nasal bone at different GA.
Foetal aneuploidy, Sonography, Ultrasound screening
Introduction
In pregnancy, the ultrasound scanning is predominantly done between 12-22 weeks to detect any structural abnormalities [1]. Early detection of abnormalities is better for both the mother and the foetus. Among the various congenital anomalies, aneuploidies are the commonly noted [2]. Previously, women >35 years used to be tested with amniocentesis for aneuploidies [3]. However, testing all women >35 years with amniocentesis was not practically possible. Therefore, foetuses were screened for the chromosomal aneuploidies with various sonographic markers, presence of which increases the risk of chromosomal abnormalities.
Down syndrome, is the most common chromosomal abnormality in neonates [4]. Previously, it was detectable in only 20-25% of foetuses using invasive prenatal diagnosis [5]. Various new tests, including improvements in ultrasonography and maternal serum biochemical studies have been reported to improve the sensitivity of screening [6-8]. Down syndrome is usually characterised by flattening of the facial profile and a small nose in neonates. On the other hand, first and second trimester sonography detects absence or hypoplasia of the foetal nasal bone in children with Down syndrome [9,10]. The detection rate of Down syndrome in second trimester was reported to be 77.7% with a false positive rate of 0.7% using the fifth percentile of foetal NBL as a cut-off value [11].
Improvements in ultrasonography made evaluating these properties easier. Often, in a clinical set up, NBL and Prenasal Thickness (PNT) are the proposed markers used for screening Down syndrome in the second trimester [12,13]. During the second trimester, NBL measurement must be performed at the exact mid-sagittal plane, with which the vomer is visualised [14,15] and any measurements obtained from parasagittal and oblique planes do not reflect true values [14].
Normal foetal NBL values have been established in Caucasian, African American and South American populations [16-18]. Higher incidence of hypoplasia of nasal bone in foetuses from healthy Asian mothers using first trimester sonography was reported [19]. Distinct ethnic differences wherein the incidence of an absent nasal bone at 11-14 weeks of gestation was higher in African Caribbean (10.4%) and Asian (6.8%) foetus than in white (2.8%) [20].
It was observed that measurement of mid-second trimester foetal nasal bone in the Chinese population was shorter than that in American and African populations [21]. Thus, the data so far has reflected an evident impact of race and ethnicity on foetal NBL. This study aimed to ascertain a reference range for second trimester NBL in healthy Indian foetus.
Materials and Methods
This cross-sectional, record based study was conducted in Sri Siddhartha Institute of Medical Sciences and Research Centre and Rajarajeshwari Medical College, Bangalore from November 2018 to April 2020. Institutional Ethical approval was obtained from both the centers for conducting this study.
Inclusion and exclusion criteria: All pregnant women (11-24 weeks of gestation) visiting the hospital for antenatal scans were included. NBL was measured in a total of 826 pregnant women. Those with abnormal karyotypes, foetal anomalies, foetal death in utero, an absent nasal bone and structural anomalies in neonates were excluded.
Foetal nasal bone was measured in a mid-sagittal view, following the method described by Sonek JD et al., [22]. The mid-sagital image plane of the foetal profile was taken with optimal magnification and the angle of insonation was 45°-135°. The maximum NBL (in millimeters) was measured using three independent images and one optimum measurement was finally considered for each patient. The ultrasound machines used were Samsung HS 70, GE Voluson S8.
The GA was calculated by the last menstrual period in women with certain and regular periods or by a first or early second trimester biometric scan [23]. In cases in which the discrepancy between the last menstrual period and sonographic dating was greater than seven days, the GA was redefined by sonographic assessment [23]. Neonatal outcomes were recorded in all cases and karyotypes were obtained from amniocentesis results in high risk cases.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Science (SPSS) version 15.0 (SPSS Inc., Chicago, IL, USA). Pearson’s correlation and linear regression analysis was used to analyse the relationship between the NBL and maternal age. The p-value <0.05 was considered statistically significant.
Results
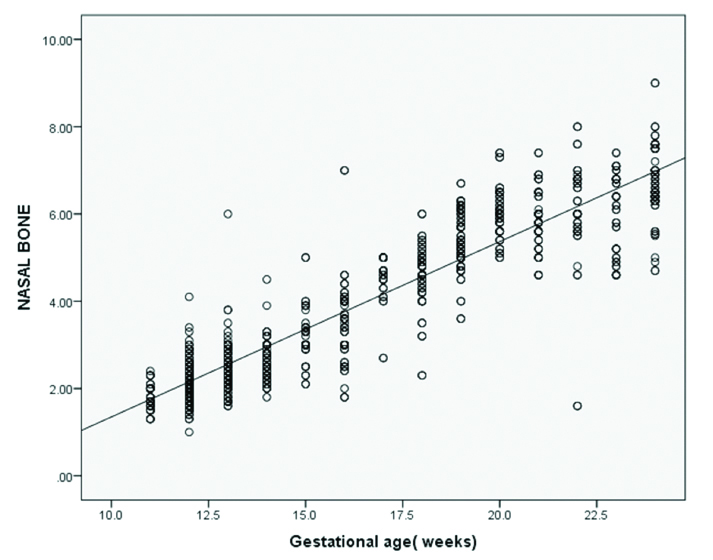
The mean maternal age was 26 years {Standard Deviation (SD): 4.3 years}. Normal nasal bone was measured at 12, 17 and 24 weeks of GA [Table/Fig-1,2 and 3]. With one unit increase in GA (weeks), NBL increased by 0.402 times (r=0.897, p<0.001). Significantly, positive correlation was seen between NBL and GA [Table/Fig-4]. The mean NBL was 4.05 mm with an overall SD of 1.18 mm. The 50th percentile NBL for each GA are shown in [Table/Fig-5]. The medians NBL for 12-14 weeks were 2.1-2.5 mm, 15-18 weeks was 3.0-4.6 mm, 19-21 weeks was 5.3-5.8 mm, 22-24 weeks was 6.0-6.6 mm [Table/Fig-5]. The NBL were comparable to the median values in Chinese [24] and Japanese [25] populations, as shown in [Table/Fig-6]. Similar comparison was seen North and South Indian population as shown in [Table/Fig-6].
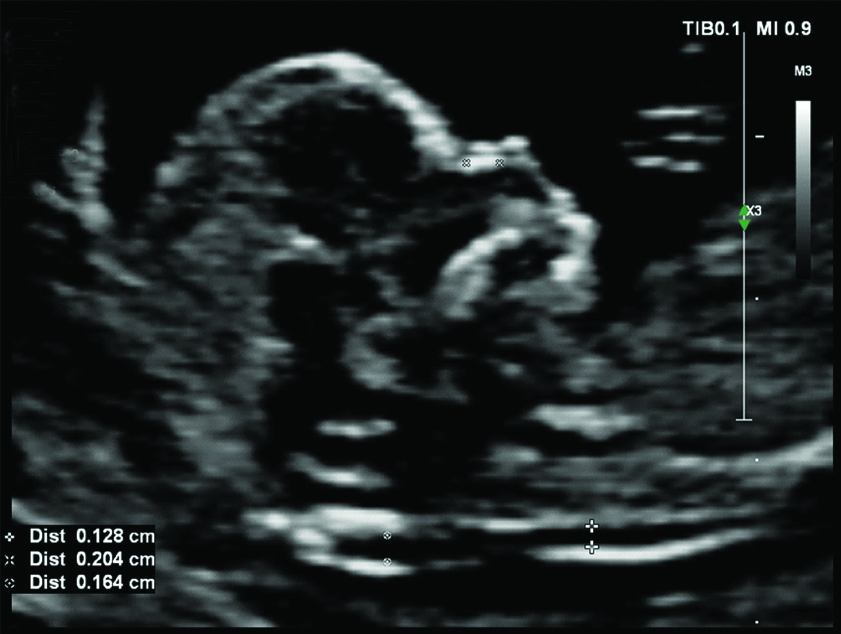
Normal nasal bone at 12 weeks GA.
GA: Gestational age
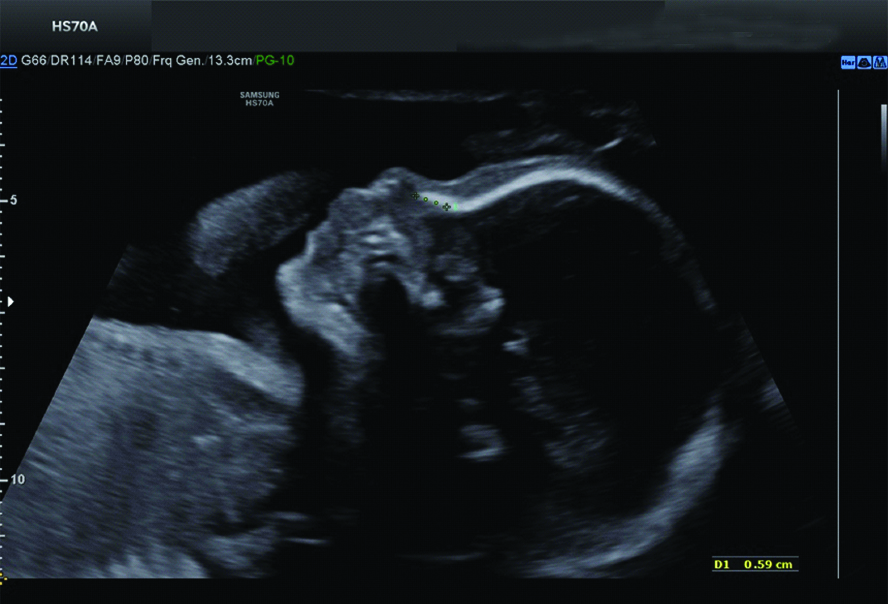
Normal nasal bone at 17 weeks GA.
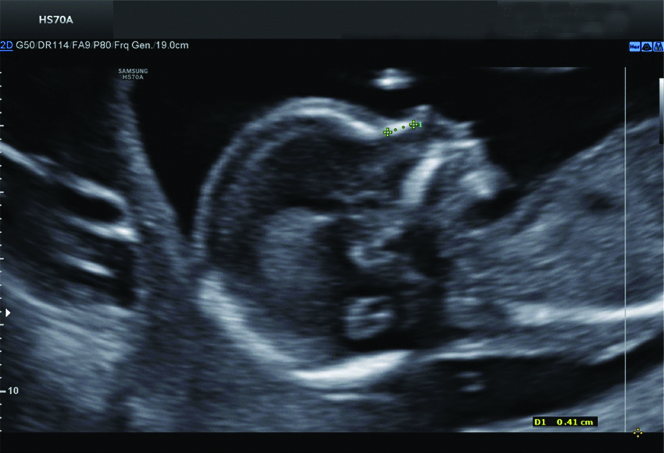
Normal nasal bone at 24 weeks GA.
Association between mean foetal NBL and GA (11-24 weeks) in the present study.
NBL: Nasal bone length
Percentile NBLs for each GA.
| Gestational age (weeks) | N | NBL (mm) by percentile |
|---|
| 5th | 10th | 25th | 50th | 75th | 90th | 95th |
|---|
| 11 | 35 | 1.30 | 1.42 | 1.60 | 1.80 | 2.00 | 2.30 | 2.32 |
| 12 | 140 | 1.50 | 1.60 | 1.80 | 2.10 | 2.50 | 2.70 | 2.90 |
| 13 | 104 | 1.70 | 1.85 | 2.10 | 2.40 | 2.88 | 3.00 | 3.28 |
| 14 | 52 | 2.00 | 2.10 | 2.30 | 2.55 | 3.00 | 3.20 | 3.51 |
| 15 | 40 | 2.11 | 2.32 | 2.93 | 3.00 | 3.48 | 3.99 | 4.95 |
| 16 | 58 | 1.99 | 2.49 | 3.00 | 3.55 | 4.00 | 4.40 | 4.72 |
| 17 | 28 | 2.70 | 3.87 | 4.30 | 4.65 | 5.00 | 5.00 | 5.00 |
| 18 | 54 | 2.98 | 3.50 | 4.20 | 4.60 | 5.00 | 5.40 | 5.63 |
| 19 | 81 | 4.05 | 4.70 | 5.00 | 5.30 | 5.90 | 6.20 | 6.30 |
| 20 | 49 | 5.05 | 5.20 | 5.60 | 5.60 | 6.45 | 6.60 | 7.35 |
| 21 | 49 | 4.60 | 5.00 | 5.50 | 5.80 | 6.50 | 6.90 | 7.15 |
| 22 | 36 | 1.60 | 4.60 | 5.60 | 6.00 | 6.80 | 7.60 | 8.00 |
| 23 | 42 | 4.60 | 4.66 | 5.15 | 6.20 | 6.80 | 7.10 | 7.36 |
| 24 | 58 | 4.89 | 5.54 | 6.30 | 6.60 | 7.28 | 7.80 | 8.05 |
NBL comparison with Asian population and other Indian population.
| GA (weeks) | 50th percentile NBL (mm) |
|---|
| Japanese population (25) | Chinese population (24) | South Indian population (21) | North Indian population (26) | Current study |
|---|
| 14 | ND | 2.9 | ND | 2.9 | 2.5 |
| 15 | 3.2 | 3.5 | ND | 3.2 | 3.0 |
| 16 | 3.5 | 4.1 | 3.3 | 3.5 | 3.5 |
| 17 | 4.5 | 4.6 | 3.7 | 4.2 | 4.6 |
| 18 | 4.9 | 5.0 | 4.2 | 4.5 | 4.6 |
| 19 | 5.2 | 5.6 | 4.6 | 4.8 | 5.3 |
| 20 | 5.8 | 5.8 | 4.9 | 5.0 | 5.6 |
| 21 | 5.7 | 6.2 | 5.3 | 5.2 | 5.8 |
| 22 | 6.6 | 6.7 | 5.7 | 5.8 | 6.0 |
| 23 | ND | ND | 6.0 | ND | 6.2 |
| 24 | ND | ND | 6.4 | ND | 6.6 |
ND: Not determined
Discussion
The NBL, an objective measurement, is a useful assessment tool in clinical practice that varies according to race and ethnicity. Absent/hypoplastic nasal bone is known to be associated with increased risk of chromosomal disease in 10-12% of cases. Similar to the second trimester maternal serum biochemical screening tests [25], ultrasound anomalies were reported in 41.4% of absent/hypoplastic nasal bone cases, and abnormal chromosomes in 28.5% of cases involving high risk biochemical studies [26]. Moreover, nasal bone can be easily visualised and measured in almost all the cases with little training and expertise, making NBL a marker for foetal aneuploidy detection.
Finding from a recent study by Cicero S et al., showed that Down syndrome was represented with an absent nasal bone or one shorter than expected for GA, making it an useful screening tool for Down syndrome in both first and second trimesters of pregnancy [9,18]. In agreement with present results, other previous studies of sonographic examinations [10,16] have shown that NBL increases with GA.
Although the mean NBL after 16 weeks gestation in this study are similar with those of Sonek JD et al., [22], the observed lengths are shorter compared with second trimester data from Brazil [16]. The attributable reasons could be due to the pronounced ethnic variability and the fact that Brazil study was conducted in a selected population of women ≥35 years of age.
In this study, successful measurement was achieved in all cases. Absence or hypoplasia of the foetal nasal bone was the single most sensitive and specific second trimester marker for Down syndrome, with a detection rate of 61.8% and a false positive rate of only 1.2% [12]. These detection rates were similar to those of second trimester maternal serum biochemical screening [25]. Although early screening of Down syndrome in neonates using NBL is of clinical importance, the issue of how the ultrasound appearance of nasal bone translates into what is seen on x-ray and on histomorphological evaluation is yet to be fully elucidated [18]. However, since these techniques cannot be used for prenatal screening and assuming that the ultrasound examination is reproducible, it remains the gold standard for prenatal screening.
Ideally, such clinically important data must be obtained in a general population whereas most of the published literature on foetal NBL throughout gestation are in patients at high risk for foetal aneuploidy [27,28]. The current study is one among the few that included normative data from 11 to 26 weeks of gestation in a general population.
Limitation(s)
Since the data was retrospectively collected in the current study, clinical implications of results presented in real time needs to be validated and captured.
Conclusion(s)
Present study findings highlight the importance of nasal bone evaluation in the second trimester which rightly captures both the phenomenon of nasal bone absence and in foetus with Down syndrome it identifies the feature of short NBL than in euploid foetus. The findings corroborate and support the sonographic assessment of NBL to prevent early chromosomal abnormalities such as Down syndrome. The results also highlight subtle, racial differences in terms of NBL at 50th percentile with other Asian population along with other Indian patient population. Studies with larger sample size are warranted to establish the correlation observed in the current setting.
ND: Not determined
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Dec 22, 2020
Manual Googling: Feb 22, 2021
iThenticate Software: Feb 19, 2021 (24%)
[1]. Karim JN, Roberts NW, Salomon LJ, Papageorghiou AT, Systematic review of first-trimester ultrasound screening for detection of fetal structural anomalies and factors that affect screening performanceUltrasound Obstet Gynecol 2017 50(4):429-41.10.1002/uog.1724627546497 [Google Scholar] [CrossRef] [PubMed]
[2]. Dey M, Sharma S, Aggarwal S, Prenatal screening methods for aneuploidiesN Am J Med Sci 2013 5(3):182-90.10.4103/1947-2714.10918023626953 [Google Scholar] [CrossRef] [PubMed]
[3]. Haddow JE, Palomaki GE, Knight GJ, Cunningham GC, Lustig LS, Boyd PA, Reducing the need for amniocentesis in women 35 years of age or older with serum markers for screeningN Engl J Med [Internet] 1994 330(16):1114-18.Available from: http://www.nejm.org/doi/abs/10.1056/NEJM199404213301603 [Google Scholar]
[4]. Cuckle H, Nanchahal K, Wald N, Birth prevalence of Down’s syndrome in England and WalesPrenat Diagn 1991 11(1):29-34.10.1002/pd.19701101061827525 [Google Scholar] [CrossRef] [PubMed]
[5]. Loncar J, Barnabei VM, Larsen JW, Advent of maternal serum markers for Down syndrome screeningObstet Gynecol Surv 1995 50(4):316-20.10.1097/00006254-199504000-000277540276 [Google Scholar] [CrossRef] [PubMed]
[6]. Wald NJ, Hackshaw AK, Combining ultrasound and biochemistry in first-trimester screening for Down’s syndromePrenat Diagn 1997 17(9):821-29.10.1002/(SICI)1097-0223(199709)17:9<821::AID-PD154>3.0.CO;2-5 [Google Scholar] [CrossRef]
[7]. Cuckle HS, van Lith JMM, Appropriate biochemical parameters in first-trimester screening for Down syndromePrenat Diagn 1999 19(6):505-12.10.1002/(SICI)1097-0223(199906)19:6<505::AID-PD572>3.0.CO;2-6 [Google Scholar] [CrossRef]
[8]. de Graaf IM, Pajkrt E, Bilardo CM, Leschot NJ, Cuckle HS, van Lith JMM, Early pregnancy screening for fetal aneuploidy with serum markers and nuchal translucencyPrenat Diagn 1999 19(5):458-62.10.1002/(SICI)1097-0223(199905)19:5<458::AID-PD569>3.0.CO;2-A [Google Scholar] [CrossRef]
[9]. Cicero S, Sonek JD, Mckenna DS, Croom CS, Johnson L, Nicolaides KH, Nasal bone hypoplasia in trisomy 21 at 15-22 weeks’ gestation: Nasal bone hypoplasia in trisomy 21Ultrasound Obstet Gynecol [Internet] 2003 21(1):15-18.Available from: http://doi.wiley.com/10.1002/uog.1910.1002/uog.1912528155 [Google Scholar] [CrossRef] [PubMed]
[10]. Bromley B, Lieberman E, Shipp TD, Benacerraf BR, Fetal nose bone length: a marker for Down syndrome in the second trimesterJ Ultrasound Med 2002 21(12):1387-94.10.7863/jum.2002.21.12.138712494981 [Google Scholar] [CrossRef] [PubMed]
[11]. Viora E, Errante G, Sciarrone A, Bastonero S, Masturzo B, Martiny G, Fetal nasal bone and trisomy 21 in the second trimesterPrenat Diagn 2005 25(6):511-15.10.1002/pd.84815968623 [Google Scholar] [CrossRef] [PubMed]
[12]. Cicero S, Curcio P, Papageorghiou A, Sonek J, Nicolaides K, Absence of nasal bone in fetuses with trisomy 21 at 11-14 weeks of gestation: An observational studyLancet 2001 358(9294):1665-67.10.1016/S0140-6736(01)06709-5 [Google Scholar] [CrossRef]
[13]. Maymon R, Levinsohn-Tavor O, Cuckle H, Tovbin Y, Dreazen E, Wiener Y, Second trimester ultrasound prenasal thickness combined with nasal bone length: A new method of Down syndrome screeningPrenat Diagn 2005 25(10):906-11.10.1002/pd.120716088862 [Google Scholar] [CrossRef] [PubMed]
[14]. Persico N, Molina F, Borenstein M, Azumendi G, Nicolaides KH, Nasal-bone length in euploid fetuses at 16-24 weeks’ gestation by three-dimensional ultrasoundUltrasound Obstet Gynecol 2010 36(3):285-90.10.1002/uog.774520623823 [Google Scholar] [CrossRef] [PubMed]
[15]. Molina F, Persico N, Borenstein M, Sonek J, Nicolaides KH, Frontomaxillary facial angle in trisomy 21 fetuses at 16-24 weeks of gestationUltrasound Obstet Gynecol 2008 31(4):384-87.10.1002/uog.528818318458 [Google Scholar] [CrossRef] [PubMed]
[16]. Bunduki V, Ruano R, Miguelez J, Yoshizaki CT, Kahhale S, Zugaib M, Fetal nasal bone length: Reference range and clinical application in ultrasound screening for trisomy 21: Fetal nasal bone lengthUltrasound Obstet Gynecol [Internet] 2003 21(2):156-60.Available from: http://doi.wiley.com/10.1002/uog.3110.1002/uog.3112601838 [Google Scholar] [CrossRef] [PubMed]
[17]. Guis F, Ville Y, Vincent Y, Doumerc S, Pons JC, Frydman R, Ultrasound evaluation of the length of the fetal nasal bones throughout gestationUltrasound Obstet Gynecol 1995 5(5):304-07.10.1046/j.1469-0705.1995.05050304.x7614133 [Google Scholar] [CrossRef] [PubMed]
[18]. Sonek JD, Nasal bone evaluation with ultrasonography: A marker for fetal aneuploidy: OpinionUltrasound Obstet Gynecol [Internet] 2003 22(1):11-15.Available from: http://doi.wiley.com/10.1002/uog.18210.1002/uog.18212858295 [Google Scholar] [CrossRef] [PubMed]
[19]. Prefumo F, Sairam S, Bhide A, Penna L, Hollis B, Thilaganathan B, Maternal ethnic origin and fetal nasal bones at 11-14 weeks of gestation: Nasal Bones And EthnicityBJOG: An International Journal of Obstetrics & Gynaecology [Internet] 2004 111(2):109-12.Available from: http://doi.wiley.com/10.1046/j.1471-0528.2003.00025.x-i110.1046/j.1471-0528.2003.00025.x-i114723746 [Google Scholar] [CrossRef] [PubMed]
[20]. Cicero S, Longo D, Rembouskos G, Sacchini C, Nicolaides KH, Absent nasal bone at 11-14 weeks of gestation and chromosomal defects: Absent nasal bone and chromosomal defectsUltrasound Obstet Gynecol [Internet] 2003 22(1):31-35.Available from: http://doi.wiley.com/10.1002/uog.17010.1002/uog.17012858299 [Google Scholar] [CrossRef] [PubMed]
[21]. Narayani BH, Radhakrishnan P, Mid-second trimester measurement of nasal bone length in the Indian populationJ Obstet Gynaecol India 2013 63(4):256-59.10.1007/s13224-012-0335-524431652 [Google Scholar] [CrossRef] [PubMed]
[22]. Sonek JD, Mckenna D, Webb D, Croom C, Nicolaides K, Nasal bone length throughout gestation: Normal ranges based on 3537 fetal ultrasound measurements: Fetal nasal bone measurementUltrasound Obstet Gynecol [Internet] 2003 21(2):152-55.Available from: http://doi.wiley.com/10.1002/uog.4110.1002/uog.4112601837 [Google Scholar] [CrossRef] [PubMed]
[23]. Butt K, Lim K, Diagnostic Imaging CommitteeDetermination of gestational age by ultrasoundJ Obstet Gynaecol Can 2014 36(2):171-81.10.1016/S1701-2163(15)30664-2 [Google Scholar] [CrossRef]
[24]. Chen M, Lee CP, Leung KY, Hui PW, Tang MHY, Pilot study on the midsecond trimester examination of fetal nasal bone in the Chinese populationPrenat Diagn 2004 24(2):87-91.10.1002/pd.79314974112 [Google Scholar] [CrossRef] [PubMed]
[25]. Kanagawa T, Fukuda H, Kinugasa Y, Son M, Shimoya K, Murata Y, Mid-second trimester measurement of fetal nasal bone length in the Japanese populationJ Obstet Gynaecol Res 2006 32(4):403-07.10.1111/j.1447-0756.2006.00429.x16882266 [Google Scholar] [CrossRef] [PubMed]
[26]. Jain S, Khanduri S, Khan M, Khan S, Yadav VK, Khan BR, Mid-second trimester measurement of nasal bone length in north Indian populationJ Clin Imaging Sci 2019 9:1410.25259/JCIS-15-201931448165 [Google Scholar] [CrossRef] [PubMed]
[27]. Odibo AO, Sehdev HM, Gerkowicz S, Stamilio DM, Macones GA, Comparison of the efficiency of second-trimester nasal bone hypoplasia and increased nuchal fold in Down syndrome screeningAmerican Journal of Obstetrics and Gynecology [Internet] 2008 199(3):281.e1-e5.Available from: https://linkinghub.elsevier.com/retrieve/pii/S000293780800711410.1016/j.ajog.2008.06.07818771983 [Google Scholar] [CrossRef] [PubMed]
[28]. Cusick W, Provenzano J, Sullivan CA, Gallousis FM, Rodis JF, Fetal nasal bone length in euploid and aneuploid fetuses between 11 and 20 weeks’ gestation: A prospective studyJ Ultrasound Med 2004 23(10):1327-33.10.7863/jum.2004.23.10.132715448323 [Google Scholar] [CrossRef] [PubMed]