Mantle Cell Lymphoma (MCL) is a rare distinctive type of NHL with unique morphologic, immunophenotypic, cytogenetic, and molecular features which forms about 5-7% of all NHL [1,2]. It is a mature B cell neoplasm believed to arise from the cells residing in the follicular mantle zones and is characterised by the genetic hallmark translocation (11;14)(q13;q32) which juxtaposes Cyclin D1 (CCND1) gene on chromosome11q13 with Immunoglobulin Heavy Locus (IGH) on chromosome 14q32, resulting in overexpression of cyclin D1 and leading to cell cycle dysregulation. CCND1/IGH translocation is seen in more than 95% of cases [3-5]. MCL can show a variety of growth patterns, and occasionally have blastoid or pleomorphic morphology with aggressive behaviour [2,3].
Recently, an indolent leukaemic non-nodal MCL and a precursor in situ mantle cell neoplasia have been included in the spectrum of mantle cell neoplasms and all of them are characterised by overexpression of cyclin D1 [1]. SOX11 is a transcription factor normally expressed in foetal brain and is involved in the pathogenesis of several tumours including B cell lymphomas. In MCL, SOX11 regulates B cell differentiation pathways and tumour microenvironment interactions and has emerged as a useful immunomarker in the diagnosis of MCL with prognostic significance [5,6]. The aim of the present study was to analyse clinicopathological and immunohistochemical features with special references to SOX11 staining and its prognostic impact in MCL. To the best of our knowledge, the present study is the largest immunomorphological study on histopathologically proven MCL cases from India and the first to evaluate SOX11 expression in the neoplastic cells.
Materials and Methods
This was a descriptive study which was carried out at a tertiary care cancer centre in Bangalore, Karnataka, India over a period of five years from January 2013 to December 2017. Clearance was obtained for the study from the Institutional Scientific and Ethical Committee (No:KMIO/MEC/017/24). There were 81 newly diagnosed cases of IHC confirmed MCL out of a total of 1397 cases of NHL forming 5.8% of NHL.
Inclusion and Exclusion criteria: Seventy six cases of MCL were included in the present study and five cases with no paraffin blocks and relapsed cases of MCL were excluded.
All the Haematoxylin and Eosin (H&E) stained slides from FFPE sections were reviewed by authors for morphologic features. IHC was carried out using Horseradish Peroxidase (HRP) polymer method, with 3,3’-diaminobenzidine tetra hydrochloride (DAB) as chromogen. FFPE tissue were sectioned at 4 micron thickness and taken on silane coated slides, dewaxed and heat induced antigen retrieval was done using the Multi-Epitope Retrieval System (MERS), blocked with 2% skimmed milk blocking solution and then incubated with a primary antibody. The bound primary antibody was detected by the addition of secondary antibody conjugated with HRP polymer and DAB chromogen. The slides were counterstained with haematoxylin and covered in a mounting medium. The following panel of antibodies were used based on the differential diagnosis on histomorphology: Leukocyte Common Antigen (LCA) (1:250, clone LJ27.9 Biogenex, Fremont CA), (Cluster of Differentiation) CD20 (1:4000, clone L26 BioSB Santa Barbara CA), CD3 (1:30, clone PS1 Biogenex, Fremont CA), CD5 (1:80, clone 4C7 Biogenex, Fremont CA), CD23 (1:100, rabbit polyclonal Biogenex, Fremont CA), Cyclin D1 (1:80, clone EP12, Biogenex, Fremont CA), SOX11 (1:100, clone C1 Pacheco CA) Ki-67 (1:100, clone MIB1 Biogenex, Fremont CA), CD10 (1:50, clone 56C6 BioSB Santa Barbara CA), BCL2 (1:200, clone BCL2/A4 BioSB Santa Barbara CA), BCL6 (1:200, clone LN22 Biogenex, Fremont CA) and TdT (1:80, clone EP266 Biogenex, Fremont CA). Positive controls from IHC proven MCL cases and negative controls with omission of primary antibody were used along with test samples.
Statistical Analysis
Data analysis was done using Statistical Package for the Social Sciences (SPSS) software version 22.0. Independent sample t-test was used to compare continuous data and Chi-square was used to test the association between categorical variables. Kaplan-Meier method of survival analysis with Log Rank test was used to compare survival curves and p-value of <0.05 was considered significant. Overall Survival (OS) was defined as the period from diagnosis to death from any cause.
Results
Demographic features: MCL formed 5.8% of NHL and there were 62 males and 14 females with an M:F ratio of 4.4:1. The patient’s age ranged from 30-87 years with a mean age of 58 years. Mean age for males was 60 years which was higher as compared to mean age for females which was 54 years. Patients more than 60 years formed 29 (38%) of cases.
Lymph nodes were the most common site involved with cervical lymphadenopathy as the most frequent presenting symptom in 58 (81.6%) cases followed by gastrointestinal tract in 12 cases (15.8%). The rarer sites of involvement included eyelid, nasopharynx, chest wall and paravertebral region. Six patients had affection of more than one site at presentation. The sites of disease at presentation are given in [Table/Fig-1].
Shows the sites of involvement of MCL at presentation.
| Sl. No. | Site | No. of cases (76)* |
|---|
| 1 | Cervical lymph node | 43 |
| 2 | Inguinal lymph node | 10 |
| 3 | Axillary lymph node | 09 |
| 4 | Epitrochlear lymph node | 01 |
| 5 | Tonsil and Waldeyer’s ring | 06 |
| 6 | Retroperitoneal lymph nodes | 02 |
| 7 | Stomach | 04 |
| 8 | Ileocecal | 01 |
| 9 | Colon | 01 |
| 10 | Rectum | 01 |
| 11 | Chest wall | 02 |
| 12 | Eyelid | 01 |
| 13 | Paravertebral mass | 01 |
*Six cases presented with involvement of more than one site
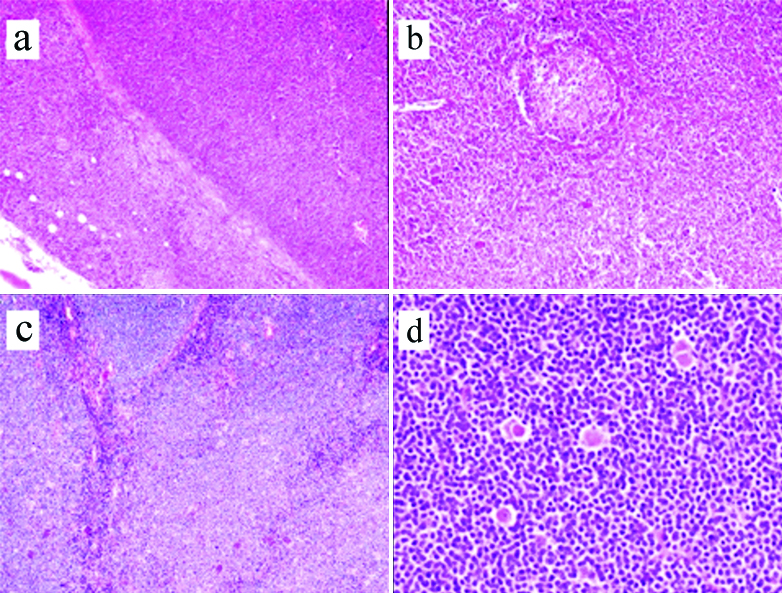
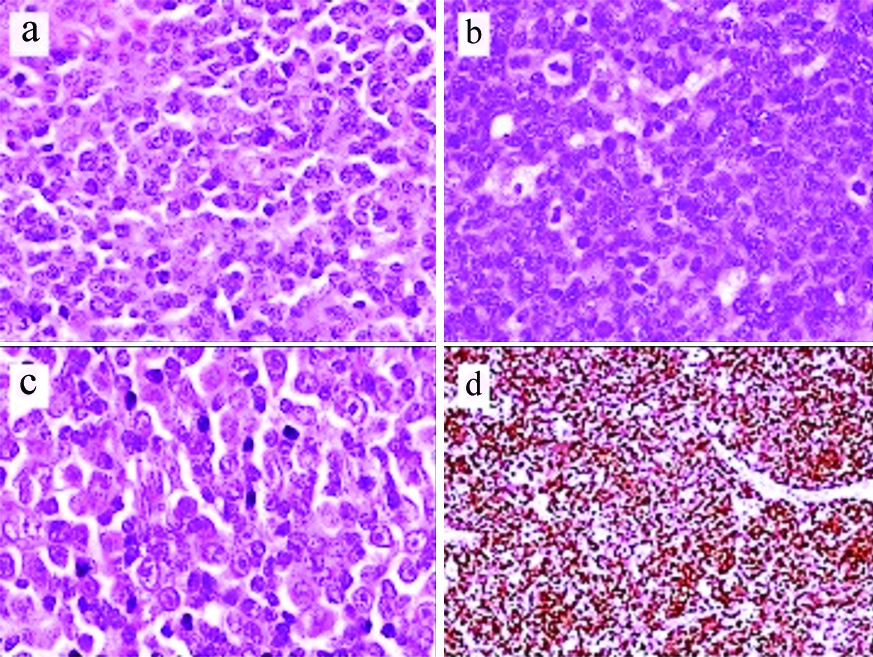
Morphology: There were 59 (77.6%) cases of classic MCL which included 13 (17.1%) with blastoid and 4 (5.3%) with pleomorphic morphology, respectively [Table/Fig-2]. Thirty six cases showed diffuse pattern (47.4%), nodular pattern was seen in 26 cases (34.2%) and mantle zone pattern of neoplastic cell infiltration was seen in eight cases (10.5%) [Table/Fig-3a-c]. Combined, diffuse and nodular pattern was seen in 2 (2.6%), 36 (47.4%), 26 (34.2%). Four (5.3%) cases could not be categorised due to scanty tumour in the biopsy. Classic MCL showed uniform small to medium sized neoplastic cells having scanty cytoplasm with irregular nuclear contours, and inconspicuous nucleoli. Frequently seen were scattered pink histiocytes which were characteristic and often gave a clue to the diagnosis of MCL [Table/Fig-3d,4a]. Thirteen (17.1%) cases had blastoid morphology with neoplastic cells having coarse nuclear chromatin and brisk mitoses (30-40 mitoses/10 hpf (high power field)), four cases (5.3%) had pleomorphic morphology with large cells having irregular nuclei and prominent nucleoli. Positive immunostaining for Cyclin D1 and SOX11 with high Ki-67 helped in establishing the diagnosis in these cases [Table/Fig-4b-d].
Clinicopathological characteristics of Mantle Cell Lymphoma (MCL).
| Variables | Number (%) |
|---|
| Age |
| Mean age-males (Mean) | 60 years |
| Mean age-females (Mean) | 54 years |
| Age >60 years | 29 (38%) |
| M:F ratio | 4.4:1 |
| Histologic subtype |
| Classic | 59 (77.6%) |
| Blastoid | 13 (17.1%) |
| Pleomorphic | 4 (5.3%) |
| Pattern |
| Diffuse | 36 (47.4%) |
| Nodular | 26 (34.2%) |
| Mantle zone pattern | 8 (10.5%) |
| Combined | 2 (2.6%) |
| Unclassified | 4 (5.3%) |
| CD5 negative | 5 (6.6%) |
| CD23 positive | 2 (2.6%) |
| Ki-67 30% or less | 24 (31.6%) |
| Ki-67 >30% | 52 (68.4%) |
| SOX11 positive | 64/69* |
| SOX11 negative | 5/69 |
| Bone marrow involved | 32 (42.1%) |
| Clinical parameters (for 33 cases) |
| B symptoms | 16 (48.5%) |
| Increased lactate dehydrogenase | 20 (60.6%) |
| Leukocyte count >10x109/L | 12 (36.4%) |
| MIPI** |
| Low risk | 11 (33.3%) |
| Intermediate risk | 10 (30.3%) |
| High risk | 12 (36.4%) |
| Stage |
| Stage 1 and 2 | 7 (21.2%) |
| Stage 3 and 4 | 26 (78.8 %) |
**MIPI: Mantle cell lymphoma international prognostic index; *IHC for SOX11 could be done on 69 cases as tissue was exhausted or not representative in seven cases
(a) Shows Mantle Cell Lymphoma (MCL) with diffuse pattern and infiltration into perinodal adipose tissue (Haematoxylin and Eosin X100); (b) Shows mantle zone pattern with residual germinal centre (Haematoxylin and Eosin X100); (c) Shows nodular pattern (Haematoxylin and Eosin X100); (d) Shows scattered pink histiocytes (Haematoxylin and Eosin X400).
(a) Shows medium sized neoplastic lymphoid cells with scant cytoplasm, irregular nuclei and inconspicuous nucleoli (Haematoxylin and Eosin X400); (b) Shows blastoid Mantle Cell Lymphoma (MCL) with cells having coarse nuclear chromatin and frequent mitoses. (Haematoxylin and Eosin X400); (c) Shows pleomorphic Mantle Cell Lymphoma (MCL) showing large neoplastic lymphoid cells many having prominent nucleoli. (Haematoxylin and Eosin X400); (d) Shows high Ki-67 proliferation in blastoid MCL (Immunoperoxidase stain X100).
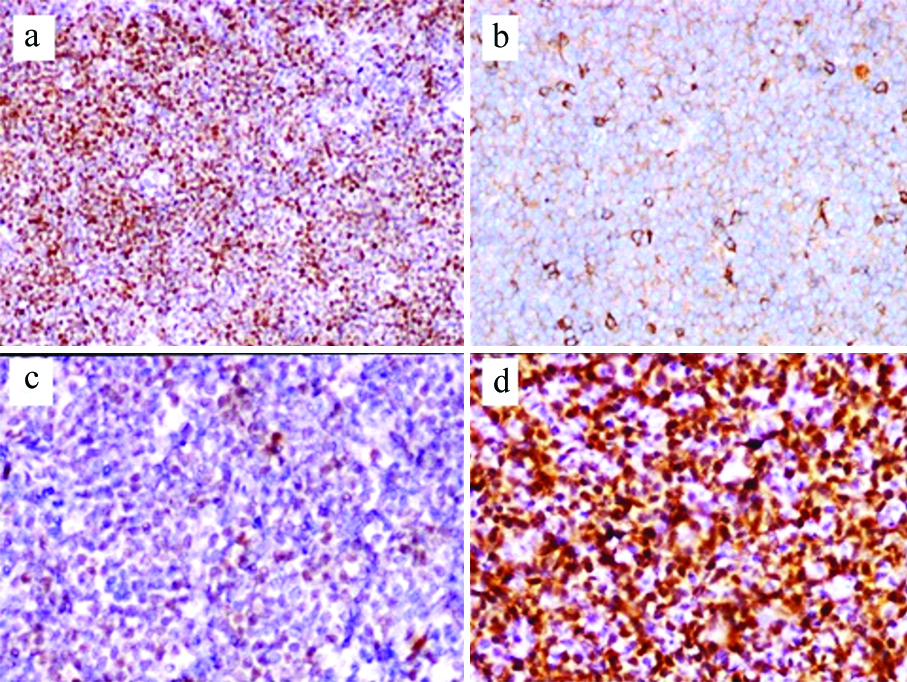
IHC findings: All 76 cases showed intense and strong expression of CD20, BCL2 and Cyclin D1 in neoplastic cells [Table/Fig-5a] with satisfactory staining in positive and negative controls. CD5 immunoreactivity was seen in all but 5 (6.6%) cases and staining was less intense as compared to adjacent T-lymphocytes [Table/Fig-5b]. IHC for SOX11 could be done on 69 cases as tissue was exhausted or not representative in seven cases. Sixty-four (92.8%) cases were SOX11 positive and 5 (7.2%) were negative. Staining for SOX11 was heterogeneous [Table/Fig-5c,d] and H-score was calculated by multiplying the intensity with percentage of positive cells and the score ranged from 10 to 240. A cut-off 100 was used to differentiate high expression from low expression. Cases with more than 50% positive cells with 2+ or 3+ intensity scored more than 100 and those with 50% or less positive cells with 2+ or 1+ intensity scored 100 or less and none with 3+ intensity were present in the latter group. A total of 34 (53.1%) had high expression and 30 (46.9%) showed low expression. Immunostaining for TdT was done in all cases with blastoid morphology to rule out lymphoblastic lymphoma and was found to be negative. CD23 was positive in two cases and CD10 was negative in all 76 cases. Ki-67 proliferation was assessed by eye balling in representative areas of atleast five high power field (hpf), according to the consensus guidelines of the European MCL network [7]. The number of pateints which had Ki-67 proliferation of 30% or less was 24 (31.6%) and 52 (68.4%) had more than 30% proliferation. Ki-67 proliferation in blastoid cases ranged from 70-90% and 60-70% in pleomorphic MCL.
(a) Shows nuclear expression of CyclinD1 (Immunoperoxidase stain X100); (b) Shows neoplastic cells with CD5 expression which is less intense than the scattered T-lymphocytes (Immunoperoxidase stain X400); (c) Shows neoplastic lymphoid cells with weak SOX11 expression (Immunoperoxidase stain X400); (d) Shows neoplastic cells with strong expression of SOX11 (Immunoperoxidase stain X400).
Bone marrow involvement: Bone marrow was involved in 32 (42.1%) cases most common pattern was diffuse infiltration (23 cases), followed by interstitial pattern of infiltration (nine cases).
Clinical details: Clinical parameters and treatment details were available in 33 cases. Out of which 7 (21.2%) patients had stage I/II disease and 26 (78.8%) patients had stage III/IV disease, of which the majority 17 (47.2%) had stage IV disease and 17 (47.2%) patients had B symptoms.
MCL International Prognostic Index (MIPI) was calculated as described by Hoster E et al., [8]. Twelve, 10 and 11 cases had high risk, intermediate risk and low risk disease respectively. Increased serum Lactate Dehydrogenase (LDH) levels were seen in 20 (60.6%) patients and 12 (36.4%) had increased total leucocyte count. The main clinicopathological features are summarised in [Table/Fig-2].
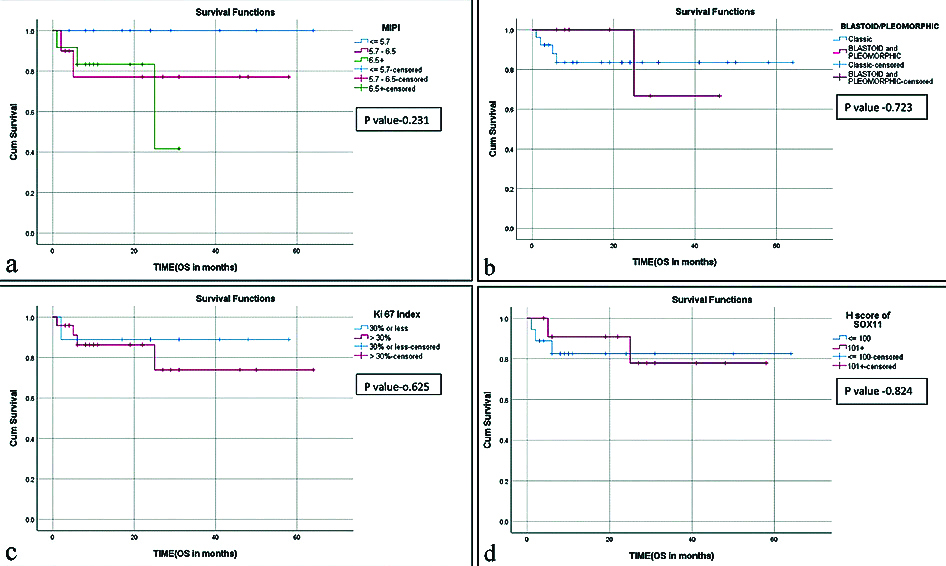
Treatment was not uniform due to differences in age, stage, symptoms and treatment era. Twenty one patients received multiagent chemotherapy with Cyclophosphamide, Doxorubicin, Vincristine, Prednisolone (CHOP), five patients received chemoimmunotherapy with four receiving Rituximab-CHOP (R-CHOP) and one Bendamustine and Rituximab (BR). Four patients received hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, cytarabine (HyperCVAD) and three patients were treated with Cyclophosphamide, Vincristine, Procarbazine, Predisolone (COPP) regimen. One patient with received Involved Field Radiotherapy (IFRT) in addition to CHOP. Six patients had complete remission, four patients had complete remission with relapse, 18 patients had partial remission and five patients died of the disease during chemotherapy. The follow-up ranged from two months to 64 months. The average OS was 21 months and high MIPI, blastoid/pleomorphic morphology and high Ki-67 were associated with poor OS [Table/Fig-6].
Kaplan-Meier survival curves for: (a) Mantle Cell Lymphoma (MCL) International Prognostic Index (MIPI); (b) blastoid/pleomorphic MCL; (c) Ki-67 proliferation index; (d) low and high expression of SOX11.
Discussion
The MCL is a mature B cell neoplasm arising from the cells residing in the follicular mantle zones and characterised by the genetic hallmark, t(11;14)(q13;q32) resulting in overexpression of cyclin D1 [1,2]. Recent studies have shown that MCL is a heterogeneous disease with a subset of leukaemic non-nodal presentation having better prognosis [2,5]. There are not many large scale studies particularly from India due to relative rarity of MCL as compared to other mature B cell lymphomas. There are several pathologic and clinical prognostic parametrers in MCL having variable results. These include pattern of growth, mitotic activity and Ki-67 proliferation, MIPI, blastoid morphology, CD 5 expression, SOX11 expression and p53 mutation [8-15]. Recent studies have focused on increased Ki-67 proliferation, SOX11 expression and p53 mutations as adverse prognostic factors [10-16].
The clinicopathological and immunophenotypic features of 76 cases of MCL were analysed. MCL formed 5.8% of cases of NHL with a male preponderance (M:F ratio 4.4:1), which is similar to various studies from India and the world literature [17-22]. The average age at presentation in the present study was 58 years (range 30 to 87 years), similar to studies from India and other Asian countries [19,21,22] while the age range was lower when compared to western literature where the mean age was around 65 years [19,23]. Interestingly, the average age for females (54 years) was lower than that for males (60 years) in present study, which was statistically significant (p-value 0.043), however there was no difference in survival (20.8 months in females vs. 20.9 months in males). Lymph node involvement (cervical followed by axillary and inguinal lymph nodes in the order of frequency) was the most common presenting feature followed by involvement of the gastrointestinal tract, which was similar to most studies [19,23,24]. Majority (78.8%) of the patients had stage III/IV disease and 22.2% had stage I/II disease with nearly half (48.5%) of the patients had B symptoms, findings similar to other studies [20-22]. MIPI is an important indicator of survival in MCL [10,13,20], in the present study MIPI was of high risk in 36.4%, intermediate risk in 30.3% of cases and low risk in 33.3% of cases. The OS was inversely proportional to the risk level (29 months in low risk vs 19.3 months in intermediate risk vs 12.8 months in high risk) though it did not reach statistical significance [Table/Fig-6a].
Morphologically diffuse involvement of the lymph nodes was the most common pattern (47.4%) followed by nodular (34.2%) and mantle zone (10.5%) patterns which did not have any prognostic significance. There were 13 cases with blastoid morphology and 4 cases with pleomorphic morphology forming 17.1% and 5.3% of cases, respectively. MCL with blastoid/ pleomorphic morphology have a poorer prognosis compared to classic MCL [20,25,26], and in present study the eight cases of blastoid/pleomorphic MCL where follow-up was available showed poor OS (17.3 months vs 28.3 months) which however did not reach statistical significance [Table/Fig-6b].
Ki-67 proliferation is one of the important indicators of prognosis with proliferation index of more than 30% being associated with poor survival [10-12]. Similar result was seen in the present study with improved OS in MCL with 30% or less Ki-67 proliferation, which however was not statistically significant [Table/Fig-6c].
SOX11 has emerged as a disease specific marker of MCL with a role in pathogenesis, diagnosis and prognosis [27]. In the 33 cases, where treatment and follow-up were available there were three SOX11 negative and 30 positive cases. As staining for SOX11 was heterogeneous with varying intensity and proportion of positive cells, authors tried to analyse the prognostic significance of high and low expression of SOX11 as determined by H score (low expression ≤100 and high expression >100) in addition to positive or negative staining for the antibody. Of the 30 positive cases high expression was seen in 12 and low expression was seen in 18 cases. However, the presence or absence of expression or the intensity of SOX11 expression had no bearing on the OS of MCL patients in the present study [Table/Fig-6d]. In the literature the prognostic significance of SOX11 expression in nodal MCL has varied with some studies showing better prognosis, some with poorer outcome and some without any prognostic significance [17,18,28-31].
There was one patient with CD5 negative MCL in the present study with follow-up, who had favorable outcome with OS of 46 months, which is consistent with findings of other studies of CD 5 negative MCL [14,32].
Limitation(s)
The limitation of the present study was lack of treatment details and follow-up of all 76 cases of MCL for analysing significant survival and prognostic indicators.
Conclusion(s)
Mantle Cell Lymphoma (MCL) is a disease of the elderly and in the present study affected females at a slightly younger age as compared to males. SOX11 immunoreactivity was complementary to cyclin D1 for diagnosis of MCL however, the intensity of expression had no effect on survival. In the 33 cases, where follow-up was available, high MIPI, blastoid/pleomorphic morphology and high Ki-67 were associated with poor OS though statistically not significant.
Funding: The study was funded by research grants from Rajiv Gandhi University of Health Sciences (RGUHS), 4th T block East, Jayanagar, Bangalore, Karnataka, India.
*Six cases presented with involvement of more than one site
**MIPI: Mantle cell lymphoma international prognostic index; *IHC for SOX11 could be done on 69 cases as tissue was exhausted or not representative in seven cases