Stroke is a leading cause of death worldwide and the third leading cause of death in western countries [1]. Carotid artery stenosis is one of the primary risk factors for stroke apart from atherosclerosis. The risk of stroke depends on the severity of the stenosis [2]. The carotid stenosis prevalence increases with age in both men and women [3]. Neurocognitive impairment and memory loss are not rare in poststroke cases. Nearly, 30% of stroke cases present with dementia within a year of stroke [4]. Stroke affects attention, memory, language, and orientation. Due to increasing life expectancy, this problem tends to become more prevalent. Even asymptomatic individuals with carotid artery stenosis may present with poor neurocognitive functions, possibly due to silent embolisation and chronic hypoperfusion. Revascularisation procedures may halt or reverse cognitive dysfunction. However, revascularisation procedures may also worsen cognitive function [3-5].
CEA is effective in reducing stroke in symptomatic as well as asymptomatic patients [4,6]. CAS is associated with an increased risk of new lesions on Diffusion Weighted Imaging (DWI) in comparison to CEA [7,8]. However, relation between CAS, revascularisation, and cognitive dysfunction remains poorly understood, with the published data showing mixed results [1,3,4,6-11]. Hence, the study was conducted to determine the effect of CAS on neurocognitive function.
Materials and Methods
This prospective observational study was carried out at tertiary care hospital, between March 2016 to March 2017, after approval (500/CA/28-26) from the the Hospital Ethics Committee. Considering a confidence level of 95% and a confidence interval of 20, the number of patients in the study to achieve statistical significance was found to be 24. This was calculated by Survey System [12]. Hence, 25 patients were randomly selected who met with inclusion and exclusion criteria.
Inclusion criteria: Patients with more than 50% carotid stenosis who were symptomatic and 70% carotid stenosis who were asymptomatic on recent Computed Tomography (CT) or Magnetic Resonance (MR) angiogram or color Doppler ultrasound study were included in the study.
Exclusion criteria: Patient with history of severe head injury, epilepsy or Intracranial Space Occupying Lesion (ICSOL) which are likely to impair cognitive function, history of mental illness or chronic alcohol abuse, non-atherosclerotic carotid stenosis, existing dementia or severe neurocognitive impairment of known aetiology other than carotid stenosis, ischaemic stroke within two weeks, vascular disease precluding catheter based techniques, intracranial aneurysm or arterio-venous malformation, history of a bleeding disorder and any surgery planned within 30 days were excluded from the study.
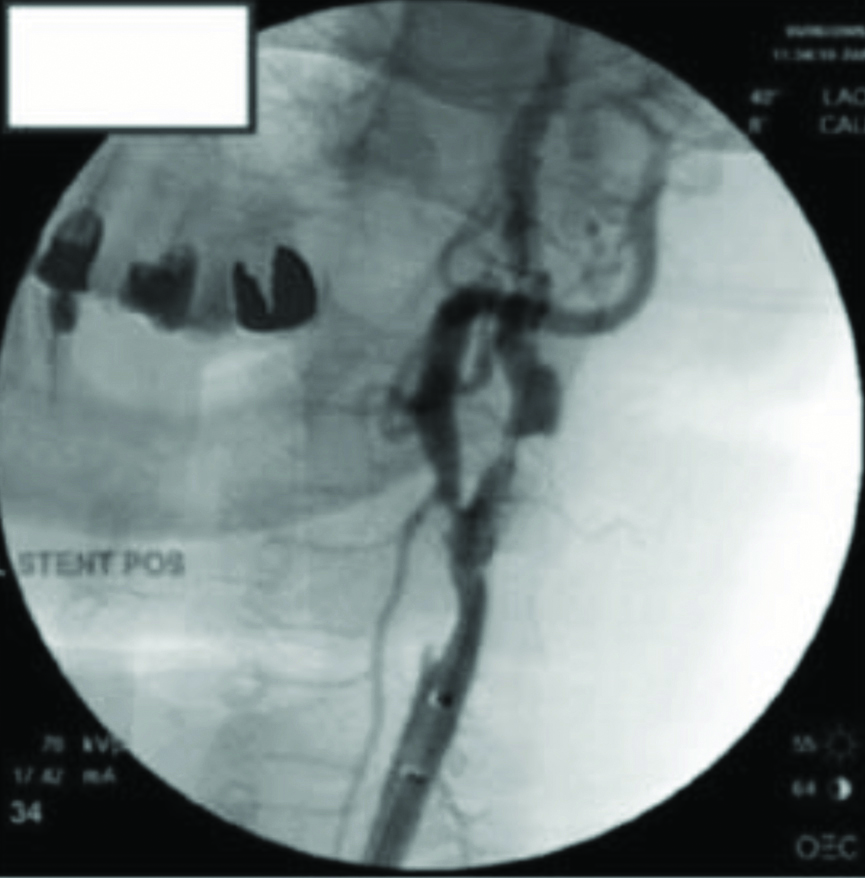
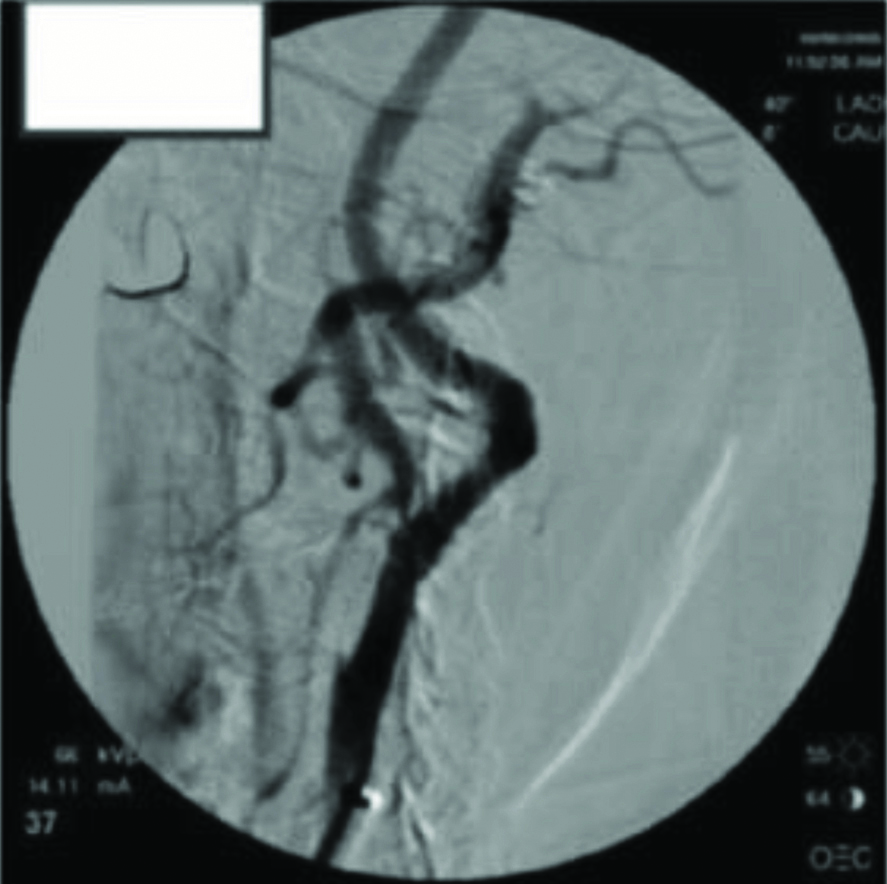
Informed consent was obtained for angiography, stenting, and neurocognitive testing. The patients underwent Addenbrooke’s Cognitive Examination Revised (ACE-R) preoperatively and were reassessed on 28 days and three months, postoperatively [13,14]. Pre and postoperative scores were compared. All these patients underwent CAS with Embolisation Protection Device (EPD) under fluoroscopic guidance by an Interventional Radiologist, with more than 15 years of experience in the field. All the patients were administered with dual antiplatelets e.g., Aspirin 75 mg and clopidogrel 75 mg OD for four days before the procedure and postprocedure dual antiplatelets were used for four weeks followed by aspirin 75 mg, which was prescribed for life long. The procedure was performed in a digital subtraction angiography lab. Local anaesthesia was given so that the patient’s neurologic status can be constantly monitored. An arch arteriogram was performed after obtaining femoral artery access. The affected side was cannulated, and selective carotid arteriograms were performed. Next, a long sheath (7 French Shuttle, Cook Medical Inc) was placed over a wire into the common carotid artery, and a 0.014-in. filter wire (EPD) was placed into the internal carotid artery distal to the lesion to provide embolic protection [Table/Fig-1]. These lesions were quickly predilated with a small balloon after appropriate size. The stent was then placed and postdilated with a larger balloon. Next, a completion arteriogram was performed to confirm that the lesion was treated and that no other abnormalities exist within the internal carotid or cerebral views [Table/Fig-2]. The procedure was completed, and the access site in the femoral artery was closed with manual pressure or a closure device. Every patient was then monitored overnight and discharged after 48 hour. All the patients included in the study were evaluated for major and minor adverse events.
Cerebral angiography showing long segment stenosis of internal carotid artery with filter wire and stent.
Postprocedure cerebral angiography showing significant improvement in diameter of internal carotid artery.
Statistical Analysis
Quantitative data was presented with the help of mean and Standard Deviation (SD). Comparison among the study groups was done with the help of the paired t-test as per the results of the normality assumption. Data analysis was done with the help of a frequency and percentage table. Association among the study groups was assessed with the help of bivariate analysis and Chi-square test and a p-value <0.05 was considered as significant. Statistical Package for the Social Sciences (SPSS) version 20.0 was used for statistical analysis.
Results
Patients included in the study were between fourth to seventh decades of life, with the majority of the patients (40%) in the age group of greater than 70 years. The mean age was 65.52±9.34 years. Twenty-two patients were males and three were females. The study group included one patient who consumed alcohol and two patients were smokers. Cerebrovascular accident (76%) was the most common coexisting co-morbidities [Table/Fig-3].
Demographic details of study population.
| Variables | Values |
|---|
| Age (Mean±SD) | 65.52±9.34 years |
| Sex | n (%) |
| Male | 22 (88%) |
| Female | 3 (12%) |
| Co-morbidities |
| Diabetes mellitus | 11 (44%) |
| Hypertension | 18 (72%) |
| CVA | 19 (76%) |
| Social history |
| Alcohol consumption | 1 (4%) |
| Smoking | 2 (8%) |
CVA: Cerebrovascular accident
Addenbrooke’s Score improved at 28 days and three months, postoperatively. There was a positive and significant correlation between preoperative Addenbrooke’s score and postoperative Addenbrooke’s score at 28 days and three months (r=0.748 and r=0.442, respectively) [Table/Fig-4a].
Correlation of pre and postoperative Addenbrooke’s score of patients.
| Addenbrook’s score | Addenbrook’s score at 28 days | Addenbrooke’s score at three months |
|---|
| Mean±SD | 75.04±9.34 | 76.92±9.46 | 79.16±9.99 |
| Paired t-test | Addenbrooke’s score at 28 days | Addenbrooke’s score at three months |
| Addenbrooke’s score | T value | 2.26 | 4.43 |
| p-value | p<0.05 | p<0.05 |
| Pearson correlation |
| Addenbrooke’s score | Pearson correlation | 0.748 | 0.442 |
| Significance (2 tailed) | p<0.05 | p<0.05 |
There was a negative correlation between age and preoperative Addenbrooke’s score (r=-0.178) and a positive correlation between age and postoperative score. There was a negative correlation between sex and preoperative Addenbrooke’s Score (r=-0.178), postoperative Addenbrooke’s Score at 28 days and three months (r=0.00 and r=0.087, respectively). But, the correlation was not statistically significant.
Co-morbid conditions showed a positive correlation on postoperative Addenbrooke’s score at 28 days and three months, but the association was not statistically significant. There was a statistically non-significant positive correlation with preoperative and postoperative Addenbrooke’s score and diabetes mellitus. However, there was a statistically non-significant negative correlation between preoperative Addenbrooke’s score and hypertension/cerebrovascular accidents [Table/Fig-4b].
Correlation of co-morbidities and pre and postoperative Addenbrooke’s scores.
| Addenbrooke score | Preoperative | At 28 days | At three months |
|---|
| DM | Pearson correlation | 0.161 | 0.395 | 0.099 |
| Sig. (2-tailed) | p>0.05 | p>0.05 | p>0.05 |
| HTN | Pearson correlation | -0.097 | 0.218 | 0.327 |
| Sig. (2-tailed) | p>0.05 | p>0.05 | p>0.05 |
| CVA | Pearson correlation | -0.031 | 0.115 | -0.115 |
| Sig. (2-tailed) | p>0.05 | p>0.05 | p>0.05 |
DM: Diabetes mellitus; HTN: Hypertension; CVA: Cerebrovascular accident
Discussion
Progressive neuropsychological cognitive deficit in patients with stenosis of the carotid artery affects a significant number of people, especially as the old age population is on the rise [10]. Patients with stenosis of these arteries show significantly poorer scores on cognitive tests [15-18]. This cognitive decline may even be a greater problem than the actual stroke, but is not a widely recognised symptom and has yet to be adequately addressed. Stent placement has been shown to have a low complication rate and evidence is accumulating that revascularisation of these arteries with the placement of stents may reduce the impaired cognitive performance [11,19,20]. This study was undertaken to measure cognitive performance prospectively before and after stent placement in patients with carotid artery stenosis.
Most of the studies as mentioned in, are suggestive of improvement in neurocognitive function post stenting, which is similar to the index study [Table/Fig-5] [20-26]. However, the study by Gaudet JG et al., reported that CAS is associated with a decline in cognitive performance immediately postoperative period [21]. The improvement in neurocognitive functioning in patients with CAS was uncertain in the studies by Zhou W et al., as these patient had high incidence of procedure associated microembolism and poor baseline cognitive function [26]. Similarly, systemic review published by Plessers M et al., and Caso V et al., so uncertain with regards to improvement in cognitive function is recorded after CEA or CAS which was either due to small and underpowered study [14,27].
Neurocognitive outcome after carotid artery stenting in various studies [20-26].
| Authors | Mean age and sample size | Associated risk factors | Results |
|---|
| Chen Y et al., [20] | 67.0±7.8 years, 240 | Smoker (23.6%)Hypertension (67.4%)Hyperlipidemia (45.8%)Atrial Fibrillation (2.8%)DM (29.2%)Prior ischaemic event (54.9%) | Global cognitive function improvement (MMSE Score-Baseline 24.6±1.7 Post six month 24.8±1.9 p-value 0.016) |
| Gaudet JG et al., [21] | 70.3±4.4 years, 24 | Hypertension (79%), Diabetes mellitus (30%), Dyslipidemia (82%), Active smoking (69%) and Oral Statin therapy (87%) | Most CAS patients appear to improve over the first month postoperatively |
| Lin MS et al., [22] | 65.8±11.5 years, 20 | Smoker (50%)Hypertension (83.0%)Hyperlipidemia (67.0%)Coronary artery disease (58%)DM (25%)Prior myocardial infarction (8%)Prior neck radiotherapy (8%)Prior ipsilateral ischaemic event (50%)NASCET symptomatic at procedure (25%) | Improved global cognitive function(MMSE Score-Baseline 25.8±3.8 Post 3 month 27.7±2.7 p-value 0.015) |
| Huang CC et al., [23] | 68.9±10.2 years, 61 | Smoker (58.6%)Hypertension (80.3%)Hyperlipidemia (63.3%)Coronary artery disease (68.3%)DM (29.3%)Peripheral arterial occlusive disease (34%)Prior myocardial infarction (12.5%)LVEF (66.33)Prior neck radiotherapy (13.5%)Prior ipsilateral ischaemic event (36%)NASCET symptomatic at procedure (25%) | Improvement in Neurocognitive function in patients with objective baseline abnormal cerebral perfusion.{MMSE Score-Baseline 27 (25-28) Post 3 month 28 (25-29) p-value 0.004} |
| Yoon BA [24] | 67.7±8.5 years, 23 | Hypertension (65.2), Diabetes mellitus (21.7), Heart disease (13.0%) and Smoking (60.9%) | Positive effect on cognitive function in patients with symptomatic carotid stenosis (K-MMSE Score-Baseline 23.5±5.3 Post 3 month difference from base line1.8±3.4 ANCOVA p-value 0.919) |
| Raabe RD et al., [25] | 73±9 years, 62 | Coronary artery disease (37±60), Diabetes mellitus (21±34), Peripheral artery disease (19±31)Values presented as means±SD | Cognitive function improved in many patients (DRS-2 total AMSS Baseline- 8.2±3.3 3 month- 9.7±3.4, 6 month- 10.0±3.7, 12 months- 10.6±3.4 Demential rating scale-revison 2 total age-corrected MOANS scaled score (DRS-2 total AMSS) |
| Zhou W et al., [26] | 71 years, 51 | Smoker (78.4%)Hypertension (96.0%)Hyperlipidemia (82.3%)Atrial fibrillation (15.6%)DM (31.3%)PVD (37.2%)Obesity (27.4%) | Neurocognitive effects post procedure remain uncertain |
PVD: Peripheral vascular disease; NASCET: North american symptomatic carotid endarterectomy trial; LVEF: Left ventricular ejection fraction; CAS: Carotid artery stenting; K-MMSE: Korean-mini-mental status examination; MOANS: Mayo older americans normative studies; AMSS: Age-corrected MOANS scaled score; DRS: Dementia rating scale
ACE-R is a brief tool for neuropsychological assessment of cognitive functions. ACE-R includes the questions from both the Mini-Mental State Examination (MMSE) and expands on the domain of memory, language, and visuospatial concepts, and adds tests of verbal fluency [13].
In the present study, there was no significant correlation between pre and postoperative Addenbrooke’s Scores with various parameters such as age, sex, and co-morbidities. It was observed that 28 days and three months after the surgery, there was improvement in Addenbrooke’s Score. There was a positive and significant correlation between preoperative Addenbrooke’s Score and postoperative Addenbrooke’s Score at 28 days and three months after operation (r=0.748 and r=0.442, respectively). Thus, successful stent placements in patients with CAS improved cognitive performance. This is in agreement with the studies of Lin MS et al., Huang C et al., and Yoon BA et al., [22-24].
Yoon BA et al., and Raabe RD et al., studied the use of comprehensive neuropsychological tests to determine the effect of CAS on cognitive function from baseline to three months post-procedure in patients with severe CS [24,25]. Of the 23 patients undergoing CAS who completed the three-month follow-up tests, 12 had asymptomatic Carotid Artery Stenosis (CS). During the follow-up period of three months, the cognitive test outcomes were similar between the patients who underwent CAS, those with asymptomatic CS and control group. Symptomatic CS patients who underwent CAS, showed significant improvements in visuospatial function and total Seoul Neuropsychological Screening Battery-Dementia Version scores (p=0.010) in comparison with both the asymptomatic CS patients and the control group [24,25]. The findings of this study implied that CAS has a positive effect on cognitive function in patients with symptomatic CS over a three-month follow-up period.
Plessers M et al., did a systematic review of the literature regarding the neurocognitive consequences of CEA and CAS [14]. The authors in the systematic review suggested that the available data showed that no obvious cognitive differences between CAS and CEA can be observed after the intervention. Caso V et al., reviewed the literature to clarify the role of carotid revascularisation on changes in cognition [27]. The authors acknowledge that certain confounding factors (learning effect, type of test, type of patients, and control group) might have influenced the assessment of cognition after carotid revascularisation. The role of carotid revascularisation in the prevention of stroke in patients with severe carotid stenosis is highlighted by previous large randomised trials [20-27]. The authors observed that although an effect of carotid revascularisation on cognition could be missed as a consequence of underpowered studies included in their review, no prediction could be done regarding its repercussions on higher intellectual functions.
Patients undergoing carotid interventions and eligible for MRI scanning were recruited in the study by Zhou W et al., [26]. Total 247 patients underwent preoperative and postoperative MRI evaluations, 51 also completed neuropsychological testing before and at one month after their procedure. The authors concluded that although CEA and CAS help in stroke prevention, with minimal neurologic complications, neurocognitive effects remain uncertain.
Raabe RD et al., carried out a study on 62 patients with significant carotid stenosis, that underwent CAS placement with embolic protection [25]. The cognitive function was assessed prospectively with the use of a battery of standardised tests administered at baseline (one to five days before CAS endovascular therapy) and at three, six, and 12 months after CAS placement. The revascularisation achieved by carotid stenting and neuro-protection resulted in either unchanged cognitive function or improved it.
Limitation(s)
The present study has limitations in the form of unavailability of the control group though patient scores after treatment were compared with scores in the same patient before treatment. This limitation may be significant because it is impossible to predict the degree to which spontaneous cognitive improvement after a stroke occurs. Another limitation is the lack of information about the cerebral perfusion state in these patients. No objective proof of alteration in perfusion exists to explain the improvement in ACE scores noted in the study. It was considered that such studies would not affect the reason for the intervention and would increase the contrast and radiation dose in the study population.
Conclusion(s)
Carotid artery stent placement for the patients with carotid stenosis who fulfill treatment criteria, improved the neurocognitive function. Further work in this area with larger patient populations is needed to confirm these findings.
CVA: Cerebrovascular accident
DM: Diabetes mellitus; HTN: Hypertension; CVA: Cerebrovascular accident
PVD: Peripheral vascular disease; NASCET: North american symptomatic carotid endarterectomy trial; LVEF: Left ventricular ejection fraction; CAS: Carotid artery stenting; K-MMSE: Korean-mini-mental status examination; MOANS: Mayo older americans normative studies; AMSS: Age-corrected MOANS scaled score; DRS: Dementia rating scale