Holoprosencephaly is a rare intracranial malformation seen in newborns, resulting from a failure of separation of the forebrain into separate cerebral hemispheres. It is divided into three subtypes-alobar, semilobar and lobar. Encephalocele is a rare neural tube defect characterised by a defect in the cranial vault with herniation of brain parenchyma along with the overlying meninges. Author has reported a case of a male neonate born of a twin pregnancy to a 29-year-old primigravida presenting with a midline swelling in the frontal region. On imaging studies, the patient was diagnosed with semilobar holoprosencephaly and frontonasal encephalocele with dural arteriovenous fistula. Holoprosencephaly and encephalocoele are important congenital malformations; however association of the two conditions has been rarely described in the literature.
Cerebral hemispheres, Intracranial malformation, Newborns
Case Report
A one-week-old male neonate born to a 29-year-old primigravida with a dichorionic diamniotic twin pregnancy, delivered at full-term by caesarian section, was referred to us. There was no history of consanguinity, congenital infection, gestation diabetes, or alcoholism. At the second trimester antenatal scan, the fetus was diagnosed with anterior encephalocele, microcephaly, and corpus callosal agenesis. The second fetus was male. The second fetus did not show any congenital anomalies on the antenatal sonography. Intranatally, there was history of meconium-stained liquor. The first neonate showed a frontal midline swelling. No other craniofacial anomalies were noted. The birth weights were 1800 and 2000 grams, respectively. Both neonates were kept in the neonatal intensive care unit for five days. The second neonate died on day five due to intracranial haemorrhage.
Magnetic Resonance Imaging (MRI) was done for the first neonate at one week of age. Frontal bony defect with herniation of brain parenchyma was demonstrated [Table/Fig-1]. Single mono-ventricle was seen; however, temporal horns were demonstrated [Table/Fig-2]. The mono-ventricle showed intra-ventricular haemorrhage with a dependent fluid-fluid level [Table/Fig-3]. Corpus callosum, septum pellucidum, and inter-hemispheric fissure were not seen. Both thalami were not seen distinctly. Brain parenchyma appeared flattened peripherally. Small focal parenchymal haemorrhages were seen in the frontal lobes [Table/Fig-3]. The posterior fossa structures including the brainstem, fourth ventricles, and cerebellum were normal [Table/Fig-4]. The binocular distance was 54 mm and the interocular distance was 18 mm.
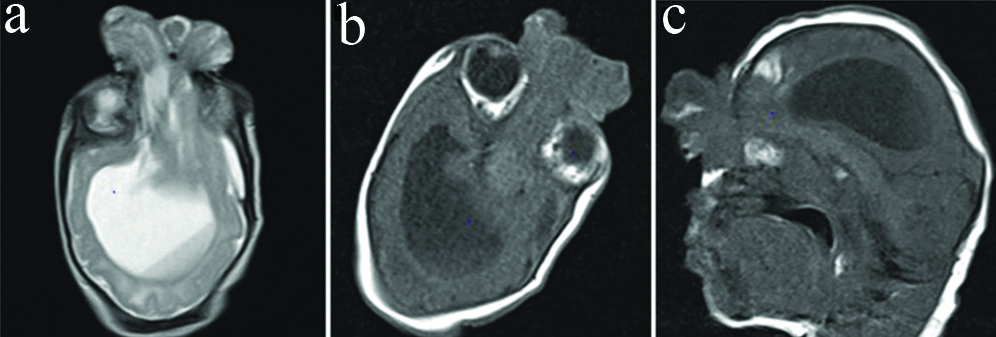
Magnetic resonance- T1 axial (a), T2 axial (b), and T1 sagittal (c) images show frontal bony defect with a herniated sac containing brain parenchyma. On T1 sagittal images, hyperintense haemorrhagic foci are seen in the frontal region.
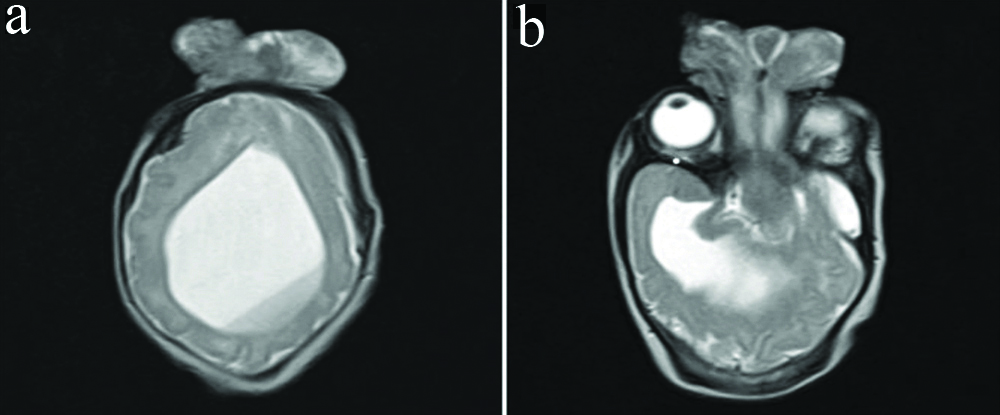
T2 weighted axial images, a single mono-ventricle is seen. Dependant fluid-fluid layering of haemorrhage is seen (a). Inferiorly a well-formed temporal horn is seen (b).
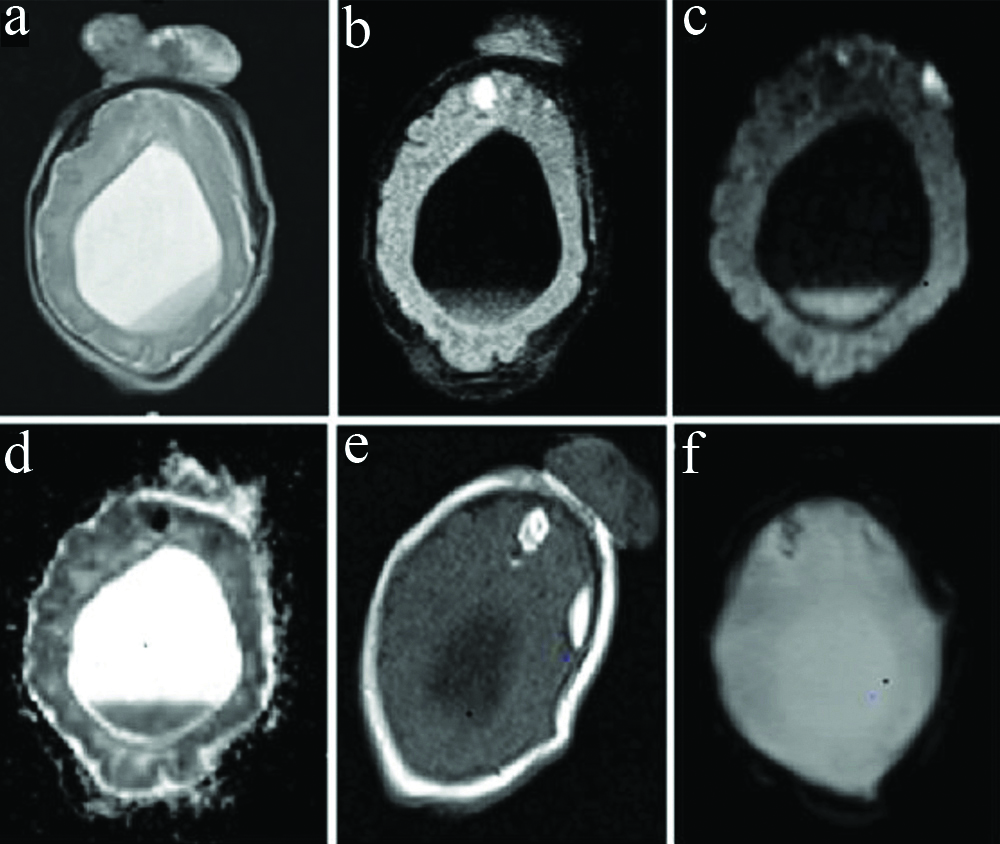
Magnetic resonance axial images-T2W (a) Fluid-attenuated inversion recovery (FLAIR) (b), diffusion-weighted (c), and apparent diffusion coefficient (d) mages show dependent fluid-fluid level with restriction on diffusion images suggestive of intraventricular haemorrhage. T1W (e) and gradient echo (f) images show haemorrhages in the frontal lobes with diffusion restriction and blooming on gradient images.
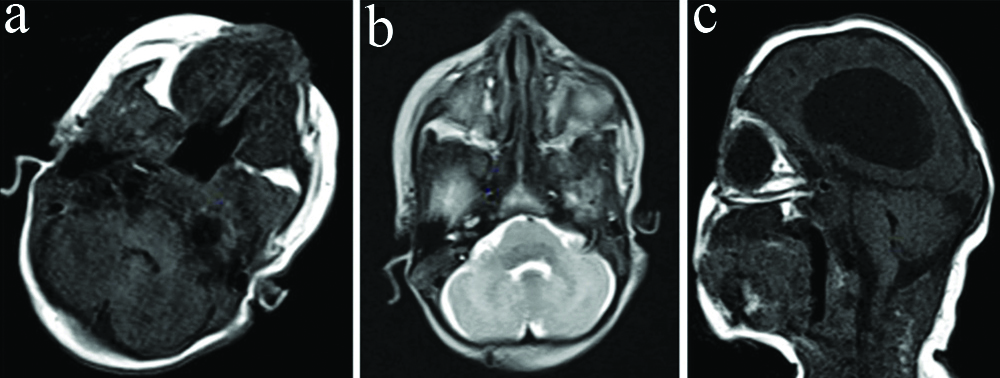
Magnetic resonance- Axial T1 (a), axial T2 (b) and sagittal T1 (c) images show normal posterior fossa structures.
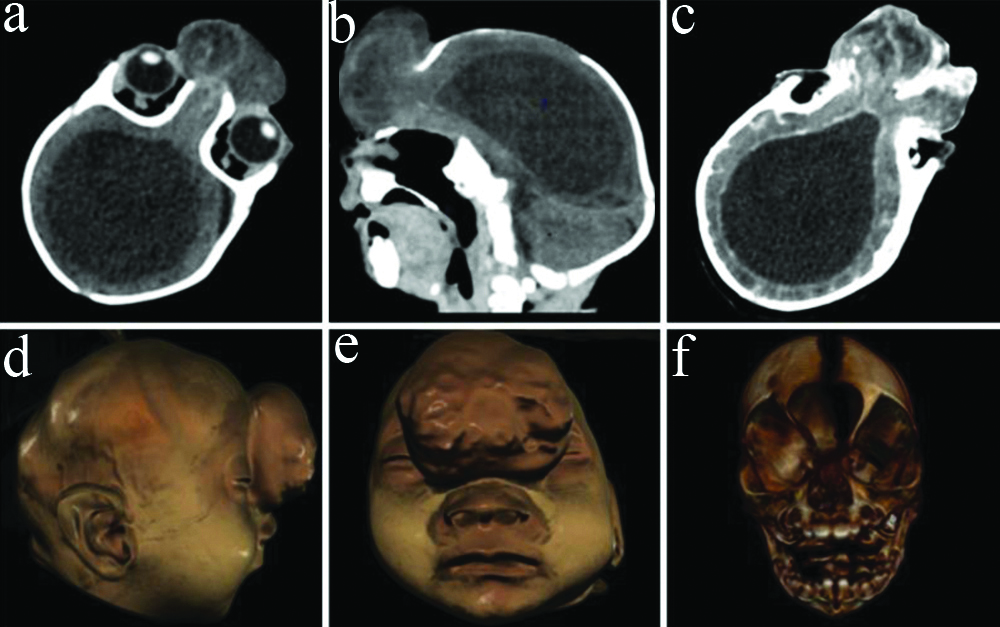
A follow-up contrast-enhanced Computed Tomography (CT) scan of the head with angiography was performed at two months. Midline frontal bony defect measuring 20×23 mm in craniocaudal and transverse dimensions was seen with herniation of brain parenchyma [Table/Fig-5]. The herniated tissue measured approximately 45×25×43 mm in craniocaudal, anteroposterior, and transverse dimensions and appeared dysplastic, showing cystic areas. The nasal bridge, cribriform plate, and olfactory grooves were not seen. A single large mono-ventricle was seen with thickened enhancing ependymal lining suggesting ventriculitis [Table/Fig-5]. The fourth ventricle was well-developed. Corpus callosum and septum pellucidum were not seen. Thalami were not seen separately. Suprasellar structures including optic chiasma were not well seen. Brain parenchyma appeared flattened peripherally. No maxillofacial abnormalities were seen [Table/Fig-5].
Computed tomography- non-contrast axial (a), reformatted sagittal (b) and contrast enhanced axial (c) images show bony defect in the frontal bone with herniated sac containing brain parenchyma and partial falx cerebri. Single mono-ventricle is seen with enhancing ependymal lining. Volume rendered sagittal (d) and coronal (e,f) images show the bony defect with frontal encephalocele and the absence of facial anomalies.
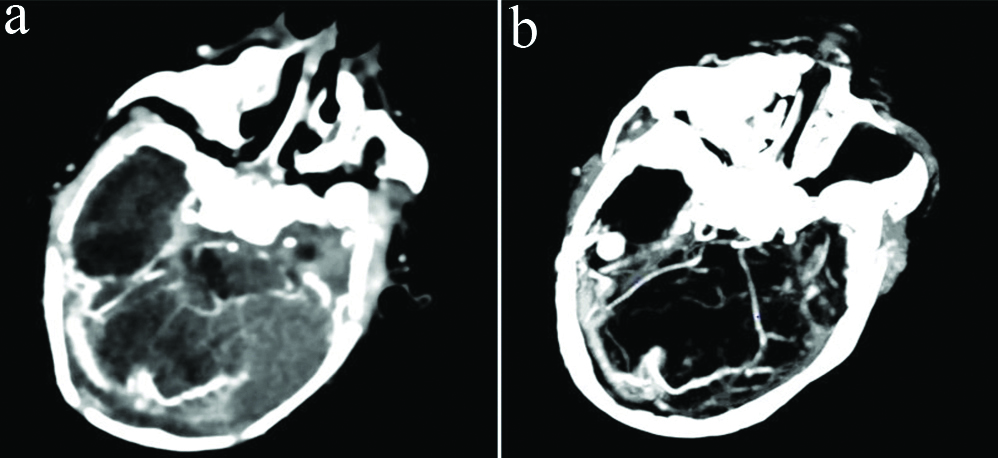
Right transverse sinus and torcular herophilli were prominent and showed early intense enhancement on angiography images [Table/Fig-6]. Prominent right posterior cerebral artery and superior cerebellar artery and their branches were seen. Arteriovenous communication was seen between the P4 segment and cortical branches of the right posterior cerebral artery and right transverse sinus.
Computed tomography-axial (a) and maximal intensity projection (b) angiography images show early intense enhancement of the right transverse sinus and torcular herophilli showing direct communication with adjacent prominent arteries, representing dural arteriovenous fistula.
The final diagnosis was given as semilobar holoprosencephaly with frontonasal encephalocele, ventriculitis, and dural arteriovenous fistula. Surgical repair of the frontonasal encephalocele was attempted at two months of age. The intraoperative gross pathology assessment revealed gliotic brain tissue and partial thinned out falx cerebri in the encephalocoele sac. Postoperatively, the infant developed sepsis and died.
Discussion
Holoprosencephaly is an uncommon brain malformation characterised by absent or incomplete fusion of the prosencephalon. Holoprosencephaly is classified into three variants based on the degree of differentiation [1-3]. Alobar holoprosencephaly is the most severe variant with absent inter-hemispheric fissure, falx cerebri, corpus callosum, sagittal sinus, and the olfactory bulbs and tracts; the fusion of thalami, basal ganglia, and cerebral hemispheres. A large crescent-shaped mono-ventricle connected to a posterior CSF-filled dorsal cyst is present. It is commonly associated with craniofacial anomalies like proboscis, cyclopia, mono-nostril, hypotelorism, and cebocephaly [2,3]. Lobar holoprosencephaly is the least severe form. The rostral and frontal portions of the frontal lobes are fused. The third ventricles and the lateral horns are well developed but the septum pellucidum is absent with dysmorphic frontal horns. The thalami and the basal ganglia are separate. Corpus callosum may be normal or hypoplastic [2,3].
Semi-lobar holoprosencephaly is intermediate in differentiation and severity, with a rudimentary inter-hemispheric fissure, incomplete falx, and anteriorly fused cerebral hemispheres. The thalami and basal ganglia show varying degrees of separation. There is a single ventricular cavity, but the lateral horns may be developed as was seen in present case. Also, the intraoperative gross pathology assessment revealed partial thinned out falx in the encephalocoele sac. A dorsal cyst may be seen. Splenium of the corpus callosum is present [2,3]. Environmental factors may include maternal diabetes mellitus, alcoholism, and TORCH (toxoplasmosis, rubella cytomegalovirus, herpes simplex, and HIV) infections [1]. There were no such risk factors in the present case. Holoprosencephaly is associated with chromosomal anomalies, most commonly trisomy 13 [4]. Genetic testing was not done in the present case.
Frontonasal encephalocele is a neural tube defect where the brain parenchyma and the overlying meninges herniate via a cranial vault defect in the region of the glabella, inferior to the frontal bones and superior to the nasal bones [5]. Jeffrey-Saint-Hillaire has proposed an embryological basis of encephalocoele formation. Since the skull is composed of two components, the endochondral cranial floor and the intra-membranous cranial vault, there is a weak point between the frontal and the ethmoidal bones in the intrauterine period when they are not fused. This weak point leads to encephalocele herniation [6]. Encephaloceles usually have very thin skin covering which may undergo dehiscence and expose the brain parenchyma to the external environment increasing the chances of neurological infections in these patients. Other complications such as haemorrhages and CSF loss can also occur [6,7]. Cerebral haemorrhages with intraventricular extent was seen in present case which was further complicated by ventriculitis. CSF leaks, wound site infection and meningitis and sepsis are the common postsurgical complication in these patients [8,9].
Meckel Gruber syndrome has holoprosencephaly as an associated finding along with the triad of occipital encephalocele, postaxial polydactyly and bilateral dysplastic cystic kidneys [10]. In present case, the other findings characteristic of Meckel Gruber syndrome were not demonstrated. Only few cases reported the association of frontonasal encephalocele with holoprosencephaly in the literature search. Cuillier F et al., reported a case of a 19-week gestation fetus with alobar holoprosencephaly, frontal encephalocele, microcephaly, and hypertelorism on antenatal ultrasound with no maternal risk factors and normal karyotype [11]. Richieri-Costa A et al., reported a case of semi-lobar holoprosencephaly, frontonasal encephaloceles, and bilateral cleft lip and palate [12]. Holoprosencephaly is found to occur more commonly in singleton pregnancies [13]. When found to occur in twin pregnancies, it often affects only one of the twin fetuses [13]. Most of the previously reported cases in twin pregnancies affected dizygotic twins [13,14].
The presence of dural arteriovenous fistula has rarely been reported in holoprosencephaly. Though, its association with encephalocele has been reported by Ahmed W et al., in a 53-year-old male patient [15]. Antenatal, ultrasonography is the primary modality to diagnosis congenital brain malformation including holoprosencephaly and encephalocele, through fetal MRI may also help in diagnosis in some patients [16,17]. Postnatally, the MRI is the imaging modality of choice to demonstrate the soft tissue details but carries the risks associated with sedation which is option required in these patients. CT is often needed to assess the bone structures and evaluate the bony defect. CT carries the risks associated with radiation exposure [18]. CT angiography may also be needed in these patients, especially to evaluate vascular anatomy and associated vascular anomalies for operative planning.
Alobar and semi-lobar holoprosencephaly are fatal in most cases, either antenatally or in the immediate postnatal period. Treatment is primarily supportive. Prognosis becomes worse when associated with other anomalies like in the present case which has associated frontonasal encephalocele and dural arteriovenous fistula. This was further complicated by the intraventricular haemorrhage and subsequently ventriculitis. Treatment for frontonasal encephalocoele consists of surgery to excise the sac, achieve complete closure of the dura, repair the bony defect and correct any facial deformities. Early surgical correction is preferred to prevent worsening of facial deformities and avoid risk of infection [13].
Conclusion(s)
The holoprosencephaly and encephaloceles are rare congenital anomalies of central nervous system, occurring separately or associated with many other anomalies. This paper highlights very rare association of semilobar holoprosencephaly with a frontonasal encephalocele and dural arteriovenous fistula in a neonate of twin pregnancy.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 24, 2020
Manual Googling: Jan 11, 2021
iThenticate Software: Feb 01, 2021 (6%)
[1]. Dubourg C, Bendavid C, Pasquier L, Henry C, Odent S, David V, HoloprosencephalyOrphanet J Rare Dis 2007 2:810.1186/1750-1172-2-817274816 [Google Scholar] [CrossRef] [PubMed]
[2]. Winter TC, Kennedy AM, Woodward PJ, Holoprosencephaly: A survey of the entity, with embryology and fetal imagingRadiographics 2015 35(1):275-90.10.1148/rg.35114004025590404 [Google Scholar] [CrossRef] [PubMed]
[3]. Osborn AG, Hedlund GL, Salzman KL, Osborn’s Brain E-Book 2017 2nd edElsevier Health Sciences [Google Scholar]
[4]. Hoving EW, Vermeij-Keers C, Frontoethmoidal encephaloceles, a study of their pathogenesisPediatric Neurosurgery 1997 27(5):246-56.10.1159/0001212629620002 [Google Scholar] [CrossRef] [PubMed]
[5]. Chen H, Chen H, Atlas of genetic diagnosis and counseling 2006 Totowa, NJHumana Press [Google Scholar]
[6]. Dhirawani RB, Gupta R, Pathak S, Lalwani G, Frontoethmoidal encephalocele: Case report and review on managementAnnals of Maxillofacial Surgery 2014 4(2):19510.4103/2231-0746.14714025593873 [Google Scholar] [CrossRef] [PubMed]
[7]. Mahajan C, Rath GP, Anaesthetic management in a child with frontonasal encephaloceleJ Anaesthesiol Clin Pharmacol 2010 26(4):570-71.PMID: 21547205; PMCID: PMC3087282 [Google Scholar]
[8]. Velho V, Naik H, Survashe P, Guthe S, Bhide A, Bhople L, Management strategies of cranial encephaloceles: A neurosurgical challengeAsian Journal of Neurosurgery 2019 14(3):71810.4103/ajns.AJNS_139_1731497091 [Google Scholar] [CrossRef] [PubMed]
[9]. Ramdurg SR, Sukanya M, Maitra J, Pediatric encephaloceles: A series of 20 cases over a period of 3 yearsJournal of Pediatric Neurosciences 2015 10(4):31710.4103/1817-1745.17446226962334 [Google Scholar] [CrossRef] [PubMed]
[10]. Kar A, Dhal I, Madurwar N, Kanungo S, Meckel-Gruber Syndrome: Autopsy based approach to diagnosisJournal of Forensic Science and Medicine 2016 2(1):5310.4103/2349-5014.165708 [Google Scholar] [CrossRef]
[11]. Cuillier F, Avignon MS, Avignon A, Alobar holoprosencephaly and frontal cephaloceleTheFetus.net c1990-2021Available from: https://sonoworld.com/TheFetus/page.aspx?id=1684 [Google Scholar]
[12]. Richieri-Costa A, Zechi-Ceide RM, Candido-Souza RM, Monteiro RA, Tonello C, de Freitas ML, Holoprosencephaly, orofacial cleft, and frontonaso-orbital encephaloceles: Genetic evaluation of a possible new syndromeAmerican Journal of Medical Genetics Part A 2019 179(11):2170-77.10.1002/ajmg.a.6130531353810 [Google Scholar] [CrossRef] [PubMed]
[13]. Idowu BM, Alobar holoprosencephaly in one of twin neonatesNew Nigerian Journal of Clinical Research 2019 8(13):35 [Google Scholar]
[14]. Burck U, Hayek HW, Zeidler U, Opitz JM, Holoprosencephaly in monozygotic twins-clinical and computer tomographic findingsAmerican Journal of Medical Genetics 1981 9(1):13-17.10.1002/ajmg.13200901047195648 [Google Scholar] [CrossRef] [PubMed]
[15]. Ahmed W, Connor S, Obholzer R, Pai I, A dural arteriovenous fistula associated with an encephalocele presenting as otitis media with effusionThe Journal of Laryngology & Otology 2018 132(11):1032-35.10.1017/S002221511800182230322412 [Google Scholar] [CrossRef] [PubMed]
[16]. Ahmed A, Noureldin R, Gendy M, Sakr S, Abdel Naby M, Antenatal sonographic appearance of a large orbital encephalocele: A case report and differential diagnosis of orbital cystic massJournal of Clinical Ultrasound 2013 41(5):327-31.10.1002/jcu.2201923203455 [Google Scholar] [CrossRef] [PubMed]
[17]. Asil K, Gunduz Y, Yaldiz C, Aksoy YE, Intraorbital encephalocele presenting with exophthalmos and orbital dystopia: CT and MRI findingsJournal of Korean Neurosurgical Society 2015 57(1):5810.3340/jkns.2015.57.1.5825674346 [Google Scholar] [CrossRef] [PubMed]
[18]. Agladioglu K, Ardic FN, Tumkaya F, Bir F, MRI and CT imaging of an Intrasphenoidal Encephalocele: A case reportPolish Journal of Radiology 2014 79:36010.12659/PJR.890795PMC4199461 [Google Scholar] [CrossRef] [PubMed]