Fracture healing is a complex process involving both anabolic and catabolic phases. There are multiple variables affecting fracture healing. These comprise of patient dependent factors, surgery related factors, medical management and rehabilitation. Many fractures are complicated by impaired healing [1]. Certain risk groups are especially predisposed to delayed union like elderly, osteoporotic patients, postmenopausal women and persons with malnutrion irrespective of mode of treatment whether conservative or surgical [1].
Many trauma patients presenting to Orthopaedic Emergency Department suffer from lower limb fractures alone or in association with other injury. These injuries lead to a prolonged period of morbidity and absence of work especially in elderly people, communited fractures or in cases with co-morbidity. To promote fracture healing supplementary vitamin D and calcium are given in prescribed dosage. In some countries, locally applied medication and electromagnetic stimulation are approved to promote fracture healing [2,3]. Parathyroid Hormone (PTH) is considered to be a potential treatment option in fractures with impaired healing [4]. TPH is a recombinant N-terminal fragment (1-34) of endogenous human PTH [5]. It has been approved by United States Food and Drug Administration (US FDA) for the treatment of postmenopausal women with osteoporosis who are at high risk for sustaining a fragility fracture. Daily treatment with TPH 20 microgram resulted in dose dependent increase in bone mineral density in lumbar spine and femoral neck in osteoporotic female and male patients [1].
Recently, role of TPH in fracture healing particularly in high risk cases to prevent and treat delayed union and non-union is being evaluated. TPH improves fracture healing by increasing endochondral ossification, bone remodeling and altering biomechanical properties of fracture callus [1]. There is a sharp rise of bone formation markers and delay of bone resorption biomarkers with use of TPH [6,7].
Huang TW et al., in a retrospective review of 255 intertrochanteric fractures (treated with daily TPH) reported shorter time to union, improved pain and function score with reduced complication rate [8]. Use of intermittent TPH is also reported to enhance callus formation in distal radius fractures and pelvic fractures [9-11]. Johansson T in a randomised control study of 40 patients treated with 20 mcg of daily subcutaneous TPH given for four weeks in proximal humerus fractures in postmenopausal women concluded no significant benefits, but did not rule out possible benefits with a different regime or in other types of fractures [12].
The TPH is still not used as a regular supplement to calcium and vitamin D in lower limb fracture cases in most of the parts of northern India; hence this study was done to determine whether TPH enhances fracture healing in case of fractures in lower limbs. Other aims were to compare function, pain and reduced need of analgesics before and after fracture treatment with TPH evaluated at regular intervals.
Materials and Methods
The present prospective interventional study was conducted on patients visiting Orthopaedic Emergency and Outpatient Department, over a period of one year from December 2019 to November 2020 at our tertiary care Government Rajindra Hospital, Patiala, Punjab, India. Sample size was calculated keeping in consideration, the number of lower limb fracture cases being treated at our institute over last two years using software G Power 3.1, effect size of 0.65 was taken. Ethical committee approval No.Trg.9 (310)2020/2617/GMC was taken and valid written consents from patients were taken. Following inclusion and exclusion criteria were involved:
Inclusion criteria: Inclusion criteria for study were fractures in lower limbs, age equal/more than 50 years, communited fractures or fractures with presence of osteopenia or osteoporosis, closed fractures. All women included were postmenopausal.
Exclusion criteria: Patients with age less than 50 years, open fractures, altered biochemical markers (calcium less than 8.6 mg/dL and/or vitamin D levels less than 20 ng/mL), presence of any metabolic bone disorder or malignancy and patients already taking TPH, patients having known malignancy, multiple injuries (more than two fractures on one lower limb, both lower limbs or injuries involving body parts in addition to lower limbs), head injury, joint disease and addiction to drug/alcohol were excluded from study.
A total of 125 patients were initially enrolled and then after meeting inclusion criteria total 104 patients were selected for study. They were divided by randomisation by 1:1 method into two groups- group A was test group of 52 cases; in which subcutaneous injection TPH 20 mcg daily (for six months) was given along with calcium 500 mg and vitamin D 25 mcg, while in 52 cases in group B (control group) only calcium 500 mg and vitamin D 25 mcg were given. Injection TPH was started within 10 days of fracture and given for six months. No placebo injection was given in control group. A further observation period of six months was included in study and final results evaluated at the end of this period.
Criteria evaluated were- time taken for radiological union; time to clinical union and full weight bearing time were noted. Clinical union was defined as absence of pain on normal loading, no abnormal movement or crepitus and no tenderness at fracture site. Radiological union is defined as formation of at least 3 out of 4 cortices on X-ray examination [13]. Pain scores by VAS on a scale of 0-100 mm [14]; at regular interval of one month, three months, six months and 12 months after treatment were also noted. In addition, DASH score was used to assess functional outcome by calculating scale scores ranging from 0-no disability to 100-most severe disability. DASH score was noted as an additional tool as DASH score questionnaire notes many activities of daily living that require mobility and stability of lower limb too [15]. Presence of any associated diabetes mellitus/and hypertension was noted.
Statistical Analysis
Data collected were entered into MS-Excel 2013 spreadsheet. The collected data were analysed using IBM SPSS version 22.0 software. Mann-Whitney U Test was used to calculate p-value. Significant p-value was taken as p-value <0.05 and data presented as mean and Standard Deviation (SD).
Results
Group A had 32 (62%) females and 20 (38%) males. Group B had 29 (56%) females and 23 (44%) males. All patients in both the groups were of more than 50 years age [Table/Fig-1]. A total of 10 (19%) cases in test group and 9 (17%) cases in control group had communited fracture [Table/Fig-2]. All fractures were of long bones i.e., femur, tibia and fibula and closed in nature [Table/Fig-3]. A 42 (40%) cases presented within 24 hours of injury, 32 (31%) cases reported between 24 to 48 hours and 30 (29%) cases came after 48 hours of injury. All patients in test group were started with TPH within 10 days of fracture. A 38/52 (73%) cases of test group while 37/52 (71%) cases of control group were treated by surgical methods by open reduction and internal fixation by interlocking nailing (42/75; 56%) and plating (33/75; 44%). All those 75 patients needing surgery in both test and control groups were operated within 2 to 7 days of presentation. None of the patients could be operated within golden period of six hours following trauma.
Age/Sex distribution of patients in both groups.
| Age group of patient (years) | Test group A | Control group B |
|---|
| 51-60 | 18 (35%) (M 8, F 10) | 17 (33%) (M 7, F 10) |
| 61-70 | 19 (36%) (M 7, F 12) | 16 (31%) (M 6, F 10) |
| More than 70 | 15 (29%) (M 5, F 10) | 19 (36%) (M 10, F 9) |
| Total | 52 (100%) | 52 (100%) |
M: Males; F: Females
Patients and fracture related characteristics in both groups.
| Characteristic | Test group A | Control group B |
|---|
| Postmenopausal women | 26/32 (81%) | 24/29 (83%) |
| Communited fractures | 10/52 (19%) | 9/52 (17%) |
| Surgically treated cases | 38/52 (73%) | 37/52 (71%) |
| Osteopenia or osteoporosis | 24/52 (46%) | 21/52 (40%) |
Long bone involved in injury in both groups.
| Bone | Test group A | Control group B |
|---|
| Femur | 32 (61%) | 30 (58%) |
| Tibia | 12 (23%) | 11 (21%) |
| Fibula | 4 (8%) | 7 (13%) |
| Both tibia and fibula | 4 (8%) | 4 (8%) |
Weight Bearing, Clinical Union and Radiological Union
Average time period for full weight bearing with support, for test group was 11±2.7 weeks and 16±1.8 weeks in control group (p=0.001). Below knee pop cast was given in 8 cases of communited fractures of tibia/fibula. Average time to clinical union in test group was 12±1.9 weeks and in control group 16±2.2 weeks. Average time to radiological union was 13±1.4 weeks in test group while 22±2.2 weeks in control group (p-0.001) [Table/Fig-4].
Average full weight bearing time and average union time in weeks (wk) in both groups.
| Variables | Test group A | Control group B | p-value | Significance |
|---|
| Weight bearing time (wk) | 11±2.7 | 16±1.8 | 0.001 | S |
| Clinical union time (wk) | 12±1.9 | 16±2.2 | 0.001 | S |
| Radiological union time (wk) | 13±1.4 | 22±2.2 | 0.001 | S |
Mann-Whitney U Test; p<0.05 significant value; S: Significant
Pain Score and Function Outcome
Decrease in pain is supposed to be an important indicator of fracture healing. On VAS from 0 to 100 mm [15], there was significant reduction in pain scores, especially on activity, in test group as compared to control group on first follow-up at one month, at three months and at six months follow-up needing fewer analgesics (p=0.001). Final follow-up at 12 months had comparable results in both groups; p>0.05 [Table/Fig-5]. DASH score was used to evaluate functional outcome. Patients treated with TPH, calcium and vitamin D had better functional outcome than those with calcium and vitamin D alone (p=0.001) [Table/Fig-6].
Level of pain (VAS score in mm) at rest and activity evaluated in both groups.
| Test group A Median (range) | Control group B Median (range) | p-value | Significance |
|---|
| At one month |
| Rest | 3 (2-36) | 8 (4-48) | 0.029 | S |
| Activity | 26 (4-67) | 42 (5-72) | 0.001 | S |
| At three months |
| Rest | 2 (1-30) | 3 (2-41) | >0.05 | NS |
| Activity | 22 (2-54) | 36 (5-69) | 0.001 | S |
| At six months |
| Rest | 0 (0-24) | 0 (0-28) | - | - |
| Activity | 11 (0-39) | 16 (1-46) | 0.001 | S |
| At 12 months |
| Rest | 0 (0-1) | 0 (0-3) | - | - |
| Activity | 3 (0-7) | 5 (0-10) | >0.05 | NS |
Mann-Whitney U Test; p<0.05 significant Value; S: Significant; NS: Non significant
DASH score (Mean) for functional outcome in both groups.
| DASH score (Mean) | Test group A | Control group B | p-value | Significance |
|---|
| Before treatment with TPH | 45 (±SD 2.72) | 46 (±SD 2.91) | >0.05 | NS |
| After treatment with TPH at 12 months | 12 (±SD 1.63) | 24 (±SD 3.45) | 0.001 | S |
Mann-whitney U Test; p<0.05 significant value; S: Significant; NS: Non significant
None of the patients were lost to follow-up due to regular and rigorous follow-up and good patient compliance. Regular treatment and follow-up resulted in good callus formation along with less adverse effects [Table/Fig-7]. There were no significant differences in adverse reactions like nausea (two each in both groups), sweating (none in both groups), headache (one each in both groups) and hypercalcaemia (one each in both groups). All of the adverse effects occurred within first month of treatment of fractures. Only two cases in test group (none in control group) had slight bruising at injection site which resolved spontaneously. There were no statistically significant fracture related other complications in both groups.
Communited fracture distal femur in 55-year-old postmenopausal woman treated with 20 mcg of subcutaneous Teriparatide (TPH) postoperatively for six months showing good union and callus formation in distal femur. a) Preoperative X-ray; b) Fracture union at six months of Teriparatide (TPH).
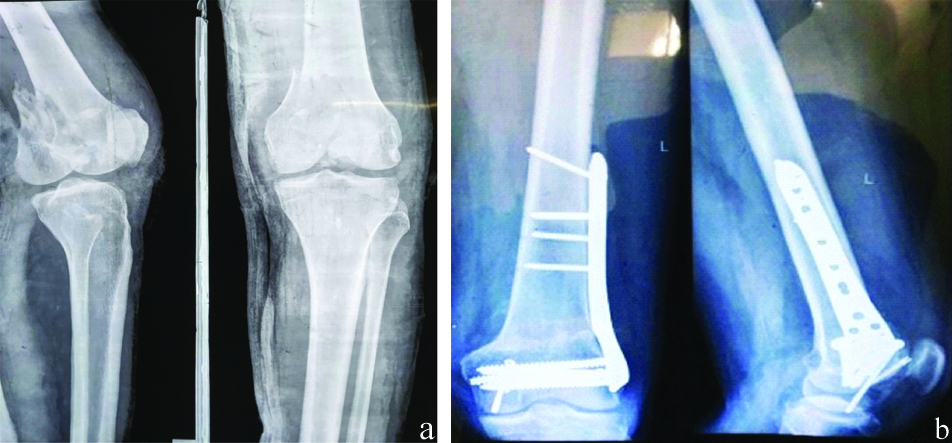
Discussion
In the present study authors have used 20 mcg of daily subcutaneous TPH for six months in lower limb fracture cases and achieved statistically significant benefits in time to clinical and radiological fracture union along with decreased pain score, better functional outcome with no significant adverse effects.
Significant reduction of clinical and radiological union time (p<0.05) was achieved in present study. Kim SJ et al., in a retrospective study observed that TPH (given daily subcutaneously in 52 of 112 patients) significantly decreased mean time to fracture healing (12.1 vs. 14.8 weeks; p=0.002) [16]. Yoon B and Kim K reported a study about radiographic features of TPH induced healing in femoral insufficiency fractures [17]. It was observed that callus was formed at very early stage, in some cases as early as two weeks. There was abundant callus formation. Normal bone remodeling was observed after one year in TPH treated cases.
In present study better pain control (p=0.001) was obtained similar to Kim SJ et al., VAS pain scores (p=0.008) [16]. Bhandari M et al., reported no differences in pain control in both groups. In present study, there was statistically insignificant difference in adverse reactions (p>0.05). Bhandari M et al., reported no differences in adverse events in both groups [18]. Almirol EA et al., and Peichi P et al., also did not report any significant adverse effects with use of TPH used in fracture healing in bones of lower limb [19,20]. [Table/Fig-8] shows adverse effects of present study compared to other studies [10,12,18,19]. Kim SJ et al., in a retrospective study observed less postoperative surgery related complications in TPH treated group [16].
Adverse effects as compared with other studies in both groups [10,12,18,19].
| Study | Adverse effect | Time | Test group n, % | Control group n, % | p-value |
|---|
| Aspenberg P et al., 2010 [10] | Serious adverse event | - | 0 (0) | 3 (8.8%) | 0.046 |
| Hypercalcaemia | - | 0 (0) | 1 | 0.490 |
| Nausea | - | 7 (40 mg group) | 0 (0) | 0.279 |
| New distal radius fracture | - | 0 (0) | 1 | 0.490 |
| Johansson T, 2016 [12] | Nausea | First 1-5 days | 0 (0%) | 3 (15.8%) | 0.160 |
| Episodes of sweating | - | 0 (0) | 2 (10.5%) | 0.260 |
| Bhandari M et al., 2016 [18] | Cases with ≥1 adverse events | - | 35 (45%) | 40 (49) | 0.634 |
| Cases with ≥1 adverse events possibly related to study drug | - | 5 (6%) | 5 (6%) | 1.000 |
| Slight headache | - | 0 (0) | 1 (5.3%) | 0.470 |
| Almirol EA et al., 2016 [19] | Slight bruising at injection site | - | 6 (100%) | 0 (0) | 0.010 |
| Pea size bump below fracture site | - | 1 (16.7%) | 0 (0) | 0.410 |
| Light headedness | - | 0 (0) | 1 (14.3) | 0.520 |
| Present study, 2021 | Nausea | First 1-10 days | 2 (3.84%) | 2 (3.84%) | 1.000 |
| Hypercalcaemia | First 1-30 days | 1 (1.92%) | 1 (1.92%) | 1.000 |
| Slight bruising at injection site (resolved spontaneously) | First 1-30 days | 2 (3.84%) | - | >0.05 |
| Headache | First 1-10 days | 1 (1.92%) | 1 (1.92%) | 1.000 |
| Sweating | - | 0 (0) | 0 (0) | - |
Mann-Whitney U Test; p<0.05 significant value
Lower limb fractures lead to considerable disability which results in loss of autonomy of patients and dependence upon other family members and relatives. Absence from work and dependence, sometimes even for daily house hold chores results in socio-economic consequences. As per Giannotti S et al., there is a negative correlation between age and fracture healing [1]. Hence, it is imperative to obtain timely clinical and radiological union in elderly. There are many factors including patient related as well as method of treatment related factors affecting various stages of callus formation in fracture healing.
The TPH treatment can amplify rate of fracture union, yet optimal dose, dose interval, timing and duration of treatment by TPH for fracture healing are yet not well-defined [21-24]. Present study was conducted to know effect of TPH in fracture healing in lower limbs. Bhandari M et al., reported that TPH remains a treatment option for hip fractures due to low trauma in elderly cases that were having high risk for further fractures [18]. Though Johansson T did not recommend TPH as a standard treatment for fractures in proximal humerus (given 20 mcg daily injection for four weeks), yet he did not rule out possibility of benefits with a different regime [12].
After excluding infection, TPH may be used even after revision surgery of non-union as per published reports by Yu W and Guo X [25]. TPH may give a further stimulus for enhanced healing in periprosthetic fractures. Though most reports have described use of daily TPH, yet a case report by Ochi K et al., has mentioned successful fusion of non-union in periprosthetic fracture after total knee replacement treated with internal fixation and twice bone graft integrated with weekly TPH [23].
Shin YH et al., reported findings supporting role of Strontium Ranelate (SR) in fracture healing and non-union [26]. A controlled trial evaluated SR in comparison to calcium and vitamin D in patients more than 60 years of age and treated conservatively. SR given in acute phases did not enhance fracture healing. In present study, TPH was given in cases more than 50 years of age and in cases treated both conservatively and surgically. All cases had adequate reduction and stable fracture fixation. Compliance of patient has to be ensured for daily injection; hence biweekly dosage regime may be tried as an alternative.
Limitation(s)
Sample size was small and heterogeneous in present study. A larger sample with multicentre trial may be included in further studies. Majority of cases were old and frail, hence needed support with a stick/walker.
Conclusion(s)
Regular intake of TPH 20 mcg subcutaneous daily given for six months can reduce time to clinical and radiological fracture union, promote early weight bearing and provide better pain control. Hence, better functional outcome and prefracture ambulatory status can be achieved with no significant adverse events.
M: Males; F: Females
Mann-Whitney U Test; p<0.05 significant value