Epilepsy, the most common neurological disorder is estimated to affect nearly 65 million population across the globe [1]. In India the prevalence of epilepsy accounts for about 1% of the population [2]. Phenytoin, a conventional antiepileptic drug is effective in the treatment of generalised tonic-clonic seizures, partial seizures and convulsive status epilepticus. Over the year’s various adverse effects of phenytoin namely phenytoin encephalopathy on chronic administration have been reported. In view of its potential adverse effects, phenytoin is not recommended as the first choice for treating epileptic seizures [3].
Hypertension, the most prevalent modifiable risk factor for both ischaemic and haemorrhagic stroke, is often associated with epilepsy [4]. Studies reveal that antihypertensives with beta receptor antagonist property such as nebivolol and propranolol possess anticonvulsant action in animal experimental models [5,6]. Nebivolol is a highly cardio selective beta blocker which is devoid of intrinsic sympathomimetic activity and a potent antioxidant and highly lipophilic drug. These properties may be useful as anticonvulsant effect which has been evaluated in previous studies, individually and in combination with lamotrigine and gabapentin [6-8].
The combined effect of nebivolol and phenytoin has not been done so far. Hence, the aim of the study was to evaluate the anticonvulsant effect of nebivolol and its combined effect with phenytoin against maximal electroshock-induced seizures in mice.
Materials and Methods
The experimental animal study was conducted in December, 2019 in the animal house at the Sri Manakula Vinayagar Medical College and Hospital, Pondicherry, India. Approval from the Institutional Animal Ethics Committee (IAEC/SMVMCH/026/2018) was obtained before starting the study. Good Laboratory Practice (GLP) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines were followed throughout the study.
Study Subjects
Thirty-six naive adult male Swiss albino mice, each weighing 20-25 g were procured from Tamil Nadu veterinary and animal sciences university, Chennai. The animals were housed in groups of six in polypropylene cages and maintained at room temperature (24-27oC) under 12:12 hour light/dark cycle and were acclimatised for a period of 7 days in the animal house prior to the experiment. The mice were put on a standard pellet diet and water ad libitum. The experiment was conducted throughout during the light period especially between 10.00 and 14.00 hours.
Drugs
Nebivolol (Nebistar, Lupin Ltd., Mumbai) at doses of 0.25 mg and 0.50 mg/kg were suspended in 0.25% of Carboxy Methyl Cellulose (CMC) in 0.9% saline solution and administered orally. The standard drug, phenytoin sodium (50 mg/mL), was procured from Pfizer, India and was diluted in 0.9% saline solution to a dose of 12.5 mg/kg. All dosages were freshly prepared on the day of the experiment. Doses were selected based on previous studies [3,6].
Experimental Design
A total 36 mice were randomly assigned to six groups. Each group comprised of six animals. The dosage and route of administration of the drugs in each group are as follows:
Group 1: Vehicle - distilled water 10 mL/kg oral
Group 2: Phenytoin 25 mg/kg Intraperitoneal (IP)
Group 3: Nebivolol 0.25 mg/kg oral
Group 4: Nebivolol 0.50 mg/kg oral
Group 5: Phenytoin 12.5 mg/kg IP and nebivolol 0.25 mg/kg oral
Group 6: Phenytoin 12.5 mg/kg IP and nebivolol 0.50 mg/kg oral.
Experimental Procedure
Animals were divided into six groups each, treated with distilled water (orally) 30 minutes before the procedure. Then MES stimuli was given using electro-convulsometer. An electrode clip was placed in the mice ear, which was moistened with saline solution before application. MES stimuli, comprising of 0.2 seconds of rectangular positive pulse (50 Ma at 50 Hz, pulse width 0.4 ms) was given and mice were observed and recorded for following parameters onset of THLE, duration of clonus, duration of THLE, number of jerks, recovery time and the percentage of protection. Mice were restrained only by hand and released at the moment of stimuli. The mice were observed for 30 minutes after MES induction. The test was considered significant, if the animal exhibited tonic extensor seizure with hind limb extension more than 90 degree from the body and sustained for more than 3 seconds following 10 seconds after stimulation [9].
Statistical Analysis
The results are expressed as mean±standard error of mean. The data was analysed using Analysis of Variance (ANOVA) followed by post-hoc Dunnett t-test. The statistical tests were done using Statistical Package for Social Sciences software, version 24.0. p<0.01 was considered statistically significant.
Results
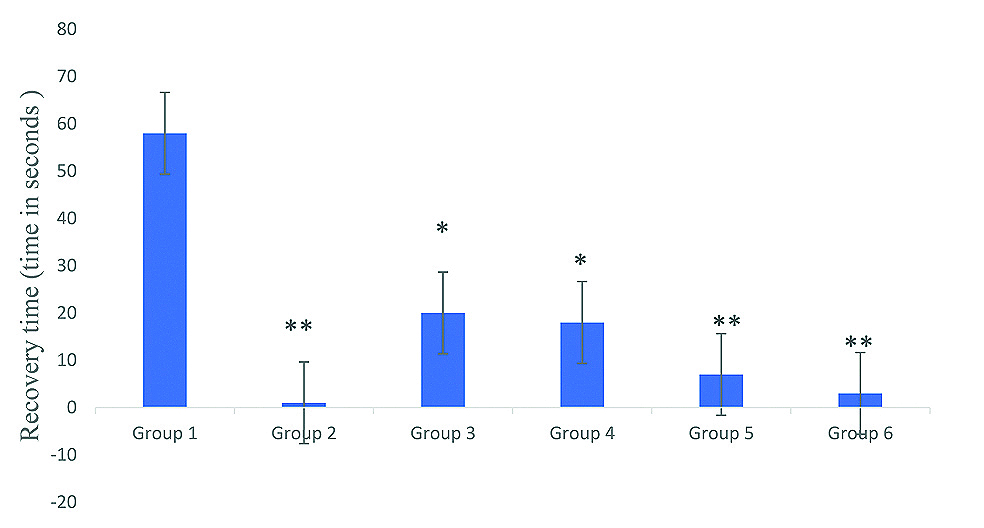
All the experimental animals chosen were weighed 20-25 g and all remained healthy at the end of the experiment. The duration of THLE was significantly decreased (p<0.01) by the combination of low dose Phenytoin (12.5 mg/kg) with Nebivolol (0.50 mg/kg) (1.27±0.307) compared to phenytoin alone (25 mg/kg) (1.33±0.333 sec). A significant (p<0.01) decrease in duration of clonus was observed with nebivolol (0.5 mg/kg) (9.33±0.615 sec). Also, when nebivolol (0.50 mg/kg) and phenytoin (12.5 mg/kg) were given in combination, a further significant (3.00±1.000 sec) (p<0.001) reduction in the duration of clonus was noted. Moreover, the combination of low dose phenytoin (12.5 mg/kg) with nebivolol (0.50 mg/kg) produced minimal number of jerks (0.83±0.477) and a significant decrease (p-value <0.01) in recovery time (3.00±0.365 sec) when compared to control group [Table/Fig-1,2].
Effect of Phenytoin and nebivolol on seizure threshold in the maximal electroshock induced seizures test in mice.
| Group | Treatment | Onset of THLE (sec) | Duration of THLE (sec) | Duration of clonus (sec) | Number of jerks |
|---|
| 1 | Distilled water 10 mL/kg oral | 2.50±0.224 | 15.83±1.376 | 13.67±0.919 | 5.00±0.730 |
| 2 | Phenytoin 25 mg/kg IP | 14.50±1.607** | 1.33±0.333** | 1.50±0.342** | 0.17±0.167** |
| 3 | Nebivolol 0.25 mg/kg oral | 4.00±0.775 | 11.33±0.843 | 10.67±0.494 | 2.50±0.671 |
| 4 | Nebivolol 0.50 mg/kg oral | 6.00±0.447 | 7.00±0.365 | 9.33±0.615* | 1.33±1.333 |
| 5 | Phenytoin 12.5 mg/kg ip+ Nebivolol 0.25 mg/kg oral | 7.33±0.333 | 3.17±0.333 | 7.50±0.847* | 1.17±0.980 |
| 6 | Phenytoin 12.5 mg/kg IP+Nebivolol 0.50 mg/kg oral | 10.83±0.749** | 1.27±0.307† | 3.00±1.000** | 0.83±0.477* |
Values are expressed as Mean±SEM. *p<0.01 compared to group I; **p<0.001 compared to Group I; †p<0.01 compared to group II; THLE: Tonic hind limb extention; n=6 in each group
Effect of phenytoin & nebivolol on recovery time of MES induced seizures test in mice.
Values are expressed as Mean±SEM; *p<0.01 compared to group 1; **p<0.001 compared to Group 1; N=6 in each group
Discussion
The anticonvulsant effect of Nebivolol and its combination with phenytoin was evaluated against Maximal Electroshock Induced Seizure (MES) in mice model. After MES reduction in duration of THLE and clonus was observed in the group treated with phenytoin (12.5 mg/kg) and nebivolol (0.50 mg/kg). Among the available models, MES is considered as the gold standard for evaluation of generalised tonic-clonic seizures on animal models of epilepsy [10]. The seizures produced by this model are highly reproducible and are electrophysiologically consistent with human seizures.
In this study, the effect on duration of tonic hind limb extension observed in combination therapy of phenytoin (12.5 mg/kg) and nebivolol (0.50 mg/kg) was less compared to the standard group phenytoin (25 mg/kg). The findings are similar to the study done by Goel R et al., on-combination therapy of nebivolol with lamotrigine. This could be due to the additive anticonvulsant action of nebivolol with phenytoin. Moreover, Goel R et al., stated, the dose of nebivolol used in combination therapy with phenytoin was 0.25 mg/kg which is about one twentieth of the human dose with anticonvulsant effect [6]. Further, the significant anticonvulsant effect of nebivolol in pentylenetetrazole induced seizure in mice by Muthukavitha G and Ahil MS, support the study findings [11].
This study results reveal that the anticonvulsant action of low dose phenytoin against MES induced seizures in mice is augmented by the potent antihypertensive drug Nebivolol. It was further explored for the prospects which could be involved in the anticonvulsant mechanism of action seen with nebivolol. One of the distinctive features of nebivolol is the vasodilatation mediated through Nitric Oxide (NO) release from endothelium [12]. Various studies have demonstrated NO to be endogenous anticonvulsant [13,14]. Moreover, the possibility of the role selective endothelial-NOS activity involved in anticonvulsant activity was studied by electrocorticographical recordings in Wistar rat [15].
Nebivolol being a potent antihypertensive, along with its beta antagonist property in anticonvulsant action, can be potentially used in polytherapy of seizure as an additive to lower the dose of phenytoin which eventually would curb the adverse effects on chronic use of phenytoin. However, further experimental studies are required to confirm the mechanism involved. Studies also reveal that the anticonvulsant property of Nebivolol in mice is attributed to the antagonistic action on beta receptor mediated activation of spontaneous epileptiform abnormalities that occurs in hippocampus [16,17].
Limitation(s)
The limitation of the study is that the chronic effect of nebivolol and its combination with phenytoin on the anticonvulsant activity was not evaluated.
Conclusion(s)
The present study concluded phenytoin could be used in submaximal doses on combination with nebivolol to produce maximal reduction in seizure induced by MES in mice. Further research through clinical trials is implicated to study the anticonvulsant property of nebivolol.
Values are expressed as Mean±SEM. *p<0.01 compared to group I; **p<0.001 compared to Group I; †p<0.01 compared to group II; THLE: Tonic hind limb extention; n=6 in each group