Head and neck cancer treatment planning technique has been moved from Three-Dimensional Conformal Radiotherapy (3DCRT) to IMRT. IMRT demonstrated superior to 3DCRT in terms of toxicity and quality of life for head and neck cancer. IMRT improves vital structure sparing like parotid glands and improvement in xerostomia with good target coverage [1-3]. Due to the use of multiple fixed beam angles in the IMRT technique, there is a significant increase in the number of MUs, as well as on BOT. Higher MUs increases the chance of secondary malignancies due to low dose irradiation and large BOT make it worsen due to intrafraction patient motion [4,5].
An optimisation algorithm is a very important part of any Treatment Planning System (TPS). Eclipse TPS (Varian Medical System) used an Analytical Anisotropic Algorithm (AAA) [13-15] for RapidArc plan calculation. The quality of RapidArc plans have been performed and checked for the different body part of tumours like brain, head and neck, lung, prostate, anal canal, and cervical tumours. Under these checked RapidArc plans found better treatment time and dose distribution with lesser MUs [16-19]. The main aim of RapidArc treatment is to provide improved target coverage along with better normal organ sparing along and lesser treatment time. Rapid deliveries provide an extra edge in patient comfort with less organ movement during the entire treatment and this has a strong clinical impact in patient treatment also. The purpose of this study was to dosimetrically investigate and compare triple arc RapidArc with nine field fixed beam angle IMRT plans in the head and neck cancer in respect of planning and dosimetric parameters.
Materials and Methods
Patient Selection, Preparation, and Dose Fractionation
Retrospectively, CT datasets of available 20 patients of SCC of the oro-pharynx and hypo-pharynx who have received radiotherapy treatment within the Department of Radiotherapy, Indraprasth Apollo Hospital, New Delhi, India, from January 2019 to December 2019 were selected for this study. Consent for radiotherapy treatment of head and neck cancer was taken from each patient as per departmental and institution protocol. [Table/Fig-1] shows data of selected patients according to disease and their staging. The study was performed following the declaration of Helsinki and all the guidelines were followed. For each patient, two different plans were generated, one by using the triple arc RapidArc technique and other by using nine fixed fields IMRT technique. Patients were immobilised with mask and scanned with a CT scanner for 3 mm slice thickness. The dose prescribed to the PTV that contains the primary tumour and lymph node (high risk) was 66 Gy at 2.2 Gy/fraction. With the PTV66 (primary and high-risk lymph node), there were two more PTVs named as PTV60 (intermediate risk) and PTV54 (low risk). These all PTVs were treated according to the Simultaneous Integrated Boost (SIB) approach in 30 fractions over six weeks, one fraction in a day, and five fractions in a week. These all doses to PTV and constraint to OARs set according to Radiation Therapy and Oncology Group (RTOG) 0022 protocol.
Tabulated form of patient according to carcinoma and staging of carcinoma tumor (T), nodes (N), and metastases (M).
| Patient number | Disease | Staging |
|---|
| 1 | Ca Oro-pharynx | T2N1M0 |
| 2 | Ca Oro-pharynx | T3N0M0 |
| 3 | Ca Oro-pharynx | T4N2M0 |
| 4 | Ca Oro-pharynx | T3N0M0 |
| 5 | Ca Oro-pharynx | T3N1M0 |
| 6 | Ca Oro-pharynx | T4N0M0 |
| 7 | Ca Oro-pharynx | T2N1M0 |
| 8 | Ca Oro-pharynx | T3N2M0 |
| 9 | Ca Oro-pharynx | T2N1M0 |
| 10 | Ca Oro-pharynx | T4N2M0 |
| 11 | Ca Hypo-pharynx | T2N1M0 |
| 12 | Ca Hypo-pharynx | T3N1M0 |
| 13 | Ca Hypo-pharynx | T2N1M0 |
| 14 | Ca Hypo-pharynx | T3N1M0 |
| 15 | Ca Hypo-pharynx | T2N1M0 |
| 16 | Ca Hypo-pharynx | T2N1M0 |
| 17 | Ca Hypo-pharynx | T3N1M0 |
| 18 | Ca Hypo-pharynx | T2N1M0 |
| 19 | Ca Hypo-pharynx | T3N1M0 |
| 20 | Ca Hypo-pharynx | T3N1M0 |
Treatment Planning and Delivery
All the treatment plans were generated by using the Eclipse TPS (Varian Medical Systems, Palo Alto, CA, USA). All the calculations were done by the High Definition (HD)-120 MLC and plans were calculated with 6 MV photons beam only. The number of fields were nine for IMRT with 600 MU/min dose rate. A triple arc technique (CW, CCW, and 90° CW) was used for the RapidArc plan. The number of control points for the RapidArc plan was 178 and at each control point, beam aperture was defined by MLC and gantry angle. Dose was delivered with a varying dose rate of 100 MU/min to 600 MU/min. The main aim of this study was systematically examine all the parameters between a RapidArc and a standard IMRT plan with clinically accepted dose distribution. Due to this a plan objective for RapidArc plan was the same as the standard IMRT plan objective for target and OARs.
Plan Evaluation Parameters
Evaluation of plans was performed by using standard Dose-Volume Histograms (DVHs). For a PTV, D98% and D2% (dose received by the 98%, and 2% of the volume) were defined as metrics for minimum and maximum doses in association to V95% and V107% (the volume receiving at least 95% or at most 107% of the prescribed dose). The target homogeneity was expressed by D5% - D95% (the difference between the dose covering 5% and 95% of the PTV). The degree of conformality of any plan was calculated by a CI. The CI (CI95%) is expressed as the ratio of the patient volume receiving at least 95% of the prescribed dose and the total volume of the PTV. For the OARs, the investigation included the mean dose, the maximum dose expressed as D2%. Additionally, the BOT and the number of MUs were analysed. The main OARs considered for all patients were submandibular glands, ipsilateral parotid, contralateral parotid, brainstem, and spinal cord. Extra OARs were contoured if it was required according to a radiation oncologist for high risk patients.
Quality Assurance
After RapidArc and IMRT treatment planning, a verification plan was generated for verification. For this different treatment plan of patients were projected to CT scan of I’MatriXX (IBA dosimetry, GmbH, Germany) planar Quality Assurance (QA) verification system [20] together with 4 cm Polymethylmethacrylate (PMMA) slabs above and beneath the active measuring area. I’MatriXX is a Two-Dimensional (2D) array of 32×32 matrixes of 1020 parallel-plate ionisation chambers. The active measuring area of I’MatriXX array is 24.4×24.4 cm. The height and diameter of each chamber are 0.55 cm and 0.4 cm, respectively. Every chamber has a sensitive volume of 0.07 cm3 and centre to centre distance between two chambers is 0.75 cm. Each one, out of 1020 chambers is read out with a custom microelectronics chip only. The isocentre is placed at the centre of the active measuring area. The 2D dose distribution in the active measuring is in frontal CT slice which was exported with a resolution of 1 mm to software OmniPro (IBA, Germany). Movie mode was selected for measurement of dose distribution and it was interpolated into the resolution of 1 mm. The analysis of results was done by using gamma analysis [21,22] for comparing measured and calculated dose distributions. For gamma evaluation, 3 mm and 3% passing criteria were selected.
Statistical Analysis
For the statistical analysis, Statistical Software Package for the Social Sciences (SPSS) version 20.0 (IBM Corporation, USA) was used. Descriptive analysis was performed to determine the mean and Standard Deviation (SD) value of different dose volume parameters of target and OARs. A paired two-tailed t-test was performed to compare the RapidArc technique with the IMRT technique for radiotherapy treatment of different head and neck cancers. The p-value <0.05 was considered for the significance of statistical inferences.
Results
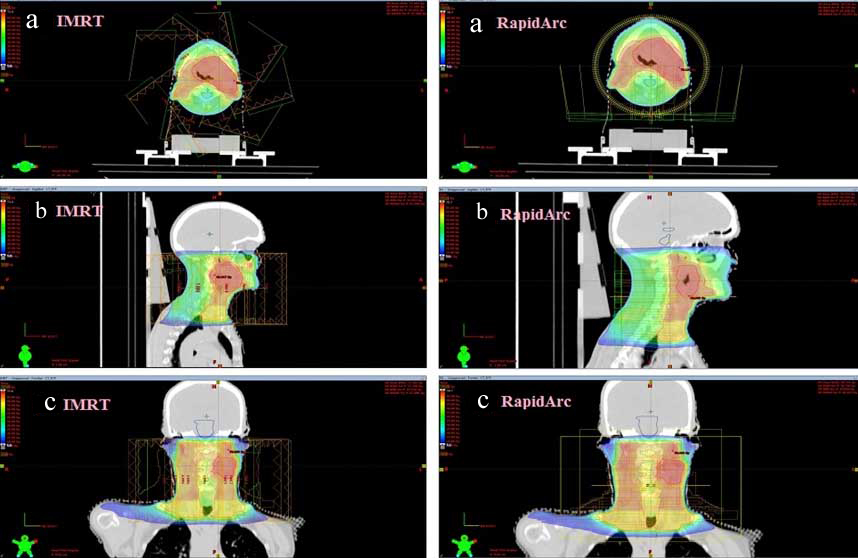
Triple arc RapidArc and nine fields IMRT plans were done for twenty patients. For each patient, two plans were generated, and hence a total of 40 plans were analysed. For comparative analysis, DVHs were generated for all the plans. [Table/Fig-2] illustrates the dose distributions for triple arc RapidArc technique and nine field IMRT techniques of a reference patient in axial, sagittal, and coronal views.
Illustration of dose distribution for radiotherapy treatment of a reference head and neck cancer patient using triple arc RapidArc technique (right side) in (a) axial view, (b) sagittal view, and (c) coronal view and similar CT cut planned by nine fields IMRT (left side).
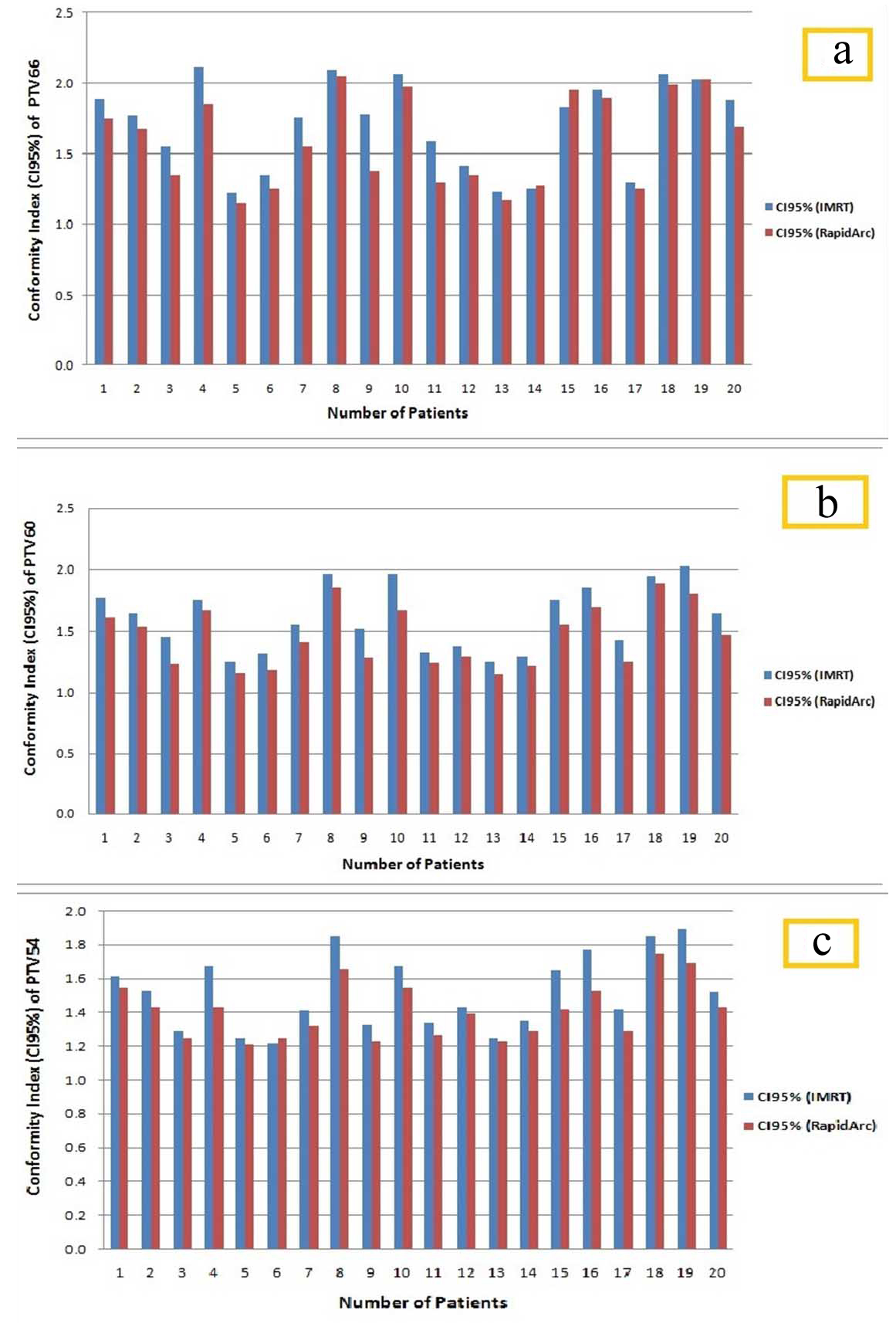
[Table/Fig-3] shows the dosimetric mean differences with their statistical results for various dose volume parameters of PTVs (PTV66, PTV60, and PTV54) between IMRT and RapidArc plans. The dosimetric parameters for PTVs were expressed as mean±SD. PTVs coverage were found nearly equivalent in both the techniques. Target homogeneity for PTV66, PTV60, and PTV54 was 9.39±0.49, 10.27±0.61, and 10.31±0.66, respectively for RapidArc plans. Target homogeneity for PTV66, PTV60, and PTV54 was 10.27±0.61, 11.49±0.62 and 11.48±0.65, respectively for IMRT plans. From the result, it is clearly showing that RapidArc plans are more homogeneous than IMRT plans (p-value <0.05). CI95% for PTV66, PTV60, and PTV54 was 1.59±0.32, 1.46±0.25, and 1.41±0.17, respectively for RapidArc plans. For IMRT technique plans, CI95% was found 1.71±0.31, 1.61±0.26, and 1.52±0.22, respectively for PTV66, PTV60, and PTV54. Dose conformity which is expressed as CI95%, the RapidArc plans give better conformity in comparison to IMRT plans (p-value<0.05) [Table/Fig-3]. [Table/Fig-4a-c] show the CI95% differences between IMRT technique and RapidArc technique plans for PTV66, PTV60, and PTV54 of individualised 20 patients, respectively.
Comparison of various dosimetric parameters for target (planning target volumes) between IMRT and RapidArc plans.
| Planning target volume | Dosimetric parameter | Mean±SD | % Mean difference | p-value(Paired t-test) |
|---|
| IMRT | RapidArc |
|---|
| PTV66 | D2% (%) | 108.47±1.55 | 106.06±1.96 | -2.22 | <0.05 |
| D98% (%) | 91.65±1.17 | 93.37±1.54 | 1.88 | <0.05 |
| D5%-D95% (%) | 10.27±0.61 | 9.39±0.49 | -8.57 | <0.05 |
| CI95% | 1.71±0.31 | 1.59±0.32 | -7.02 | <0.05 |
| PTV60 | D2% (%) | 108.47±1.47 | 106.05±1.96 | -2.42 | <0.05 |
| D98% (%) | 91.97±1.08 | 93.71±1.51 | 1.89 | <0.05 |
| D5%-D95% (%) | 11.49±0.62 | 10.27±0.61 | -10.62 | <0.05 |
| CI95% | 1.61±0.26 | 1.46±0.25 | -9.32 | <0.05 |
| PTV54 | D2% (%) | 108.47±1.48 | 106.06±1.95 | 2.22 | <0.05 |
| D98% (%) | 89.95±1.32 | 91.65±1.17 | 1.89 | <0.05 |
| D5%-D95% (%) | 11.48±0.65 | 10.31±0.66 | -10.19 | <0.05 |
| CI95% | 1.52±0.22 | 1.41±0.17 | -7.24 | <0.05 |
*p<0.05 significant
Illustration of Conformity Index (CI95%) comparisons between IMRT and RapidArc plans of individualise patients for PTV 66 (a), PTV 60 (b), and PTV 54 (c).
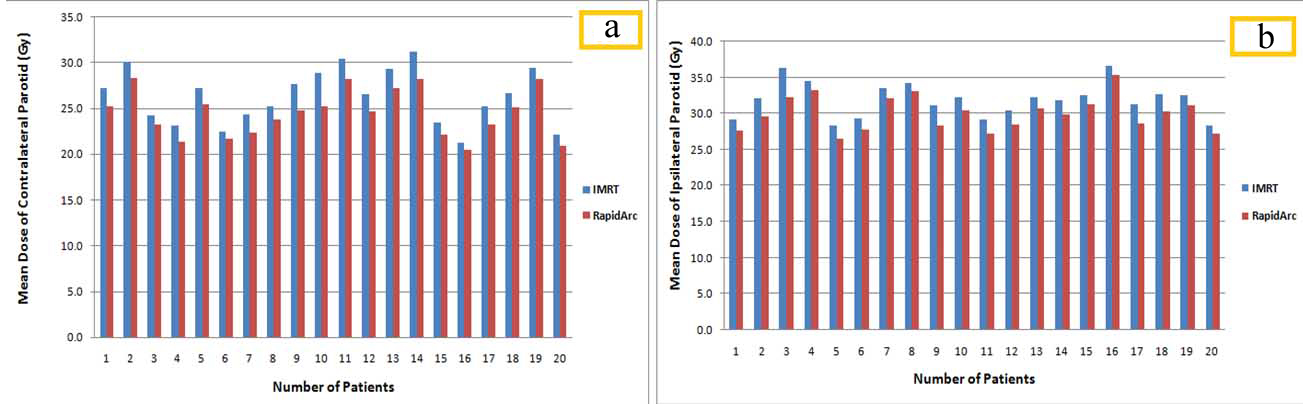
[Table/Fig-5] shows the dose differences (Mean±SD) with their statistical results of various dosimetric parameters for OARs. All OARs sparing objectives were achieved by both the planning techniques. No big difference was seen in maximum, minimum, and mean doses. But RapidArc gives better results in respect of IMRT in the entire format. Both the planning techniques were able to achieve maximum dose planning objective of 45 Gy to the spinal cord. Planning constraints for brainstem was also achieved by both the techniques but better in the RapidArc plan (p-value<0.05) [Table/Fig-5]. The analysis for contra and ipsilateral parotids were carried out separately. It is the main OARs for such type of cases and sparing is also difficult with maintaining PTV coverage and all. Due to this a detailed analysis was done by evaluating different parameters like mean dose, V5Gy, V20Gy, and V30Gy for both parotids. By comparing, a significant difference was observed between RapidArc technique plans and IMRT technique plans with better sparing in the RapidArc plans for all the parameters of OARs except the V30Gy of spinal cord (p-value=0.420) and V5Gy of ipsilateral parotid (p-value=0.450) [Table/Fig-5].
Comparison of various dosimetric parameters for organs at risk between IMRT and RapidArc plans.
| Organs at risk | Dosimetric parameter | Mean±SD | % Mean Difference | p-value |
|---|
| IMRT | RapidArc |
|---|
| Spinal cord | Mean (Gy) | 31.23±3.25 | 28.98±2.85 | -7.0% | <0.05 |
| Max (Gy) | 44.12±0.41 | 43.21±0.86 | -2.0% | <0.05 |
| D2% (Gy) | 42.74±0.67 | 41.7±1.09 | -2.4% | <0.05 |
| V30Gy | 0.33±0.087 | 0.32±0.079 | -3.03% | 0.420 |
| Brain stem | D2% (Gy) | 36.19±8.45 | 31.98±8.43 | -11.6% | <0.05 |
| Contralateral parotid | Mean (Gy) | 26.35±2.98 | 24.53±2.58 | -6.91% | <0.05 |
| V5Gy | 0.91±0.072 | 0.88±0.075 | -3.3% | <0.05 |
| V20Gy | 0.51±0.15 | 0.48±0.14 | -5.9% | <0.05 |
| V30Gy | 0.35±0.087 | 0.313±0.089 | -10.57% | <0.05 |
| Ipsilateral parotid | Mean (Gy) | 31.95±2.39 | 30.08±2.38 | -5.85% | <0.05 |
| V5Gy | 0.97±0.16 | 0.96±0.14 | -1.03% | 0.450 |
| V20Gy | 0.59±0.13 | 0.56±0.13 | -5.1% | <0.05 |
| V30Gy | 0.49±0.13 | 0.47±0.13 | -4.1% | <0.05 |
| Sub-mandibular glands | Mean (Gy) | 57.45±3.91 | 55.21±3.75 | -3.9% | <0.05 |
| V25Gy | 0.98±0.13 | 0.96±0.12 | -2.04% | <0.05 |
| V30Gy | 0.94±0.11 | 0.92±0.11 | -2.13% | <0.05 |
| V54Gy | 0.54±0.15 | 0.51±0.15 | -5.56% | <0.05 |
[Table/Fig-6a,b] illustrate the mean dose differences between IMRT technique plans and RapidArc technique plans for contra and ipsilateral parotids of individualised 20 patients, respectively. Like the parotids, Authors evaluated submandibular glands and detailed analysis was performed by including many parameters such that mean dose, V25Gy, V35Gy, and V54Gy. For both the techniques satisfactory result with no much differences was obtained but definitely better sparing in RapidArc plan (p-value <0.05) [Table/Fig-5]. The final module of the clinical execution of RapidArc was the analysis of patient specific QA and how it affects the clinical workflow. In terms of the effect on workflow, RapidArc plans must be recalculated on a phantom, which will take longer time than the IMRT procedure. However, a portion of this time was compensating as the delivery will be faster for RapidArc. It was also important to understand what the QA results before treating patients clinically. By using the passing criteria of gamma analysis (dose difference of 3% and a distance-to-agreement of 3 mm), patient specific QA results were similar for IMRT technique and RapidArc technique plans. The average passing rate was 94.6% and 97.5% for the IMRT plans and RapidArc plans, respectively.
Mean dose differences between IMRT and RapidArc plans for (a) Contralateral parotid and (b) Ipsilateral parotid.
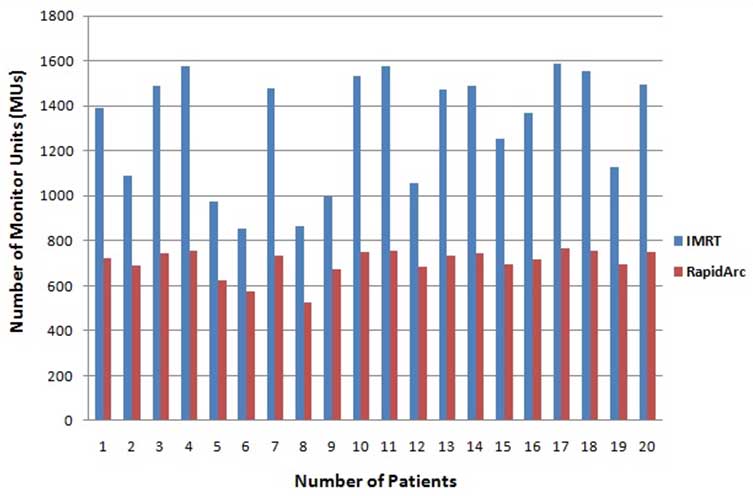
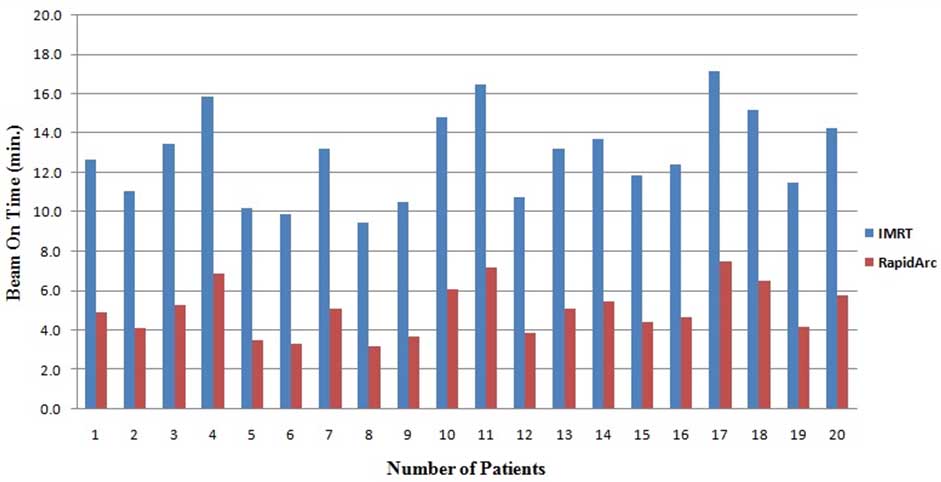
[Table/Fig-7] shows the comparison of planning parameters (MUs and BOT) between treatment plans generated with the IMRT technique and RapidArc technique. These parameters were expressed as mean±SD. The number of MUs per fraction (2.2 Gy/fraction) for IMRT and RapidArc plans was 1310±257 and 704±64, respectively. IMRT plans show that the values of MUs were approximately double (46%) in comparison to the RapidArc plan (p-value <0.05) [Table/Fig-7]. [Table/Fig-8] illustrates the number of MUs for both planning techniques (IMRT and RapidArc) of individualised 20 patients. Treatment time for IMRT was found to be higher due to split fields and dead times such as the times required for reposition and reprogram the linear accelerator for every field. Due to this entire BOT for IMRT technique plans was approximately double (46%) than the RapidArc plans (p-value <0.05). [Table/Fig-9] illustrates the BOT for IMRT and RapidArc techniques plans of individualised patients.
Monitor units and beam on time for triple arc RapidArc and nine fields IMRT.
| Planning parameter | Mean±SD | % Mean difference | p-value(Paired t-test) |
|---|
| IMRT | RapidArc |
|---|
| Number of Monitor Units (MUs) | 1310±257 | 704±64 | -46.26% | <0.05 |
| Beam on time (min) | 12.9±2.26 | 5.1±1.31 | -39.53% | <0.05 |
Comparison of monitor units between IMRT technique and RapidArc technique plans.
Beam on time comparison between IMRT technique and RapidArc techniques plans.
Discussion
There is more uniform dose distribution on surface in RapidArc techniques, due to multiple beam angles. In comparison of RapidArc, IMRT provide intensity modulation in only selected beam angle only, due to this, it needs more static fields to achieve same dose distribution as RapidArc technique. A multiple arc technique provides more control point in comparison of multiple static field IMRT techniques. Lin MH et al., explained that RapidArc was more suitable technique for centrally located target and IMRT was best suited for peripheral target [23]. But comparative plan was possible in both the techniques. A methodical study of these treatment techniques for different sites can provide precious information. Treatment planning studies for the different sites have been studied by several researchers in the interest of comparison for RapidArc with conventional IMRT techniques [5,24,25]. They reported that all the plans were comparable but RapidArc was faster and uses fewer MUs for treatment. In the present study, authors did not consider single arc techniques because in many studies it has been reported that single arc plans are inferior to multiple arc plans in comparison to tumour coverage, homogeneity, conformity, and critical structure sparing [26,27]. The result of this study was in favour of the RapidArc plans with coverage of 95% isodose line. The critical structure sparing and dose homogeneity is again in favour of RapidArc plans with more than 50% lesser MUs. Smet S et al., study reported that the grade of acute toxicity was lower for the RapidArc plan in comparison to the IMRT plan [28]. It is debatable whether single, double, or multiple arcs should be applied to realise proper volumetric modulated arc techniques. The solution to this issue cannot be exploited with single study since the need for complex modulation patterns depends on several factors, mostly linked to the clinical indication. Results showed that multiple arcs can improve both the sparing of OARs and target coverage.
Generally, it is difficult to compare plans in respect of patient population, OARs contouring, and due to different type of accelerators used in the study. But Vanetti E et al., study reported a large reduction in OARs doses during transforming from IMRT to RapidArc plan by comparing DVHs [29]. Verbakel WF et al., study reported that more than 600 MUs reductions per fraction, when transforming from IMRT plan to RapidArc plan [5]. According to Kry SF et al., study, less MUs can reduce the chances of secondary malignancies but the exact calculation of risk is not feasible [30]. Bertelsen A et al., study investigated dose and distance difference test which are more sensitive to setup error than gamma evacuation and their result showed that RapidArc plans are less sensitive to small setup errors [27]. Smet S et al., study retrospectively compared IMRT and RapidArc plans and found that the target coverage with 95% of the prescribed dose is in favour of RapidArc Plan with better dose homogeneity and good sparing of OARs [28]. They also reported a more than 62% reduction in MUs. However in the present study, the delivery efficiency of the RapidArc plans was higher in terms of fewer MUs and especially in reduced BOT.
Limitation(s)
The planning rules were applied as similar as possible between techniques, dose calculation algorithm, and evaluation tools were unified but a lot of care should be taken for minimising arbitrary elements. But it is impossible to completely control all potential sources of bias and their effect on planning results comparison. In general, these limitations are common in the comparative planning study. Less BOT and effective treatment time have a very strong impact on treatment. This should be further investigated systematically on a large number of groups. Also, it was a retrospective study on 20 patients with head and neck cancer. So a prospective study should be done on larger data set for the clinical validation of the results of this study.
Conclusion(s)
RapidArc with three arc techniques offer a potential advantage over nine field fixed angle IMRT techniques. The eclipse TPS produced a good RapidArc plan with better target coverage and better conformity. The planning objective values for OARs were in general improved when shifting from IMRT techniques to RapidArc techniques. When comparing the RapidArc plans to the IMRT plans, the high dose volumes in the healthy tissue were reduced on an average, but the low dose volumes were increased. The delivery efficiency of the RapidArc plans was higher in terms of fewer MUs and especially in reduced BOT. In conclusion, RapidArc plans generated by the Eclipse TPS are clinically acceptable compared to standard IMRT. However, further investigations are required to verify whether the dosimetric and delivery advantages can be translated into benefits in clinical outcomes.
*p<0.05 significant