The Gastric Schwannoma (GS) is a rare gastrointestinal mesenchymal tumour which arises from Schwann cells in neural plexus of gastric wall. It accounts for 2-7% of gastric mesenchymal tumours, 4% of benign tumours in stomach and 0.2% of all gastric tumour [1-4]. Daimaru Y et al., described the first series of 24 cases of immunohistochemically documented GS [5]. Few case reports of GS have been described in Indian literature [6-8]. In this study, eight cases of GS were analysed over a period of 10 years. GS occurs mostly in middle aged females with mean age of 58 years [4,5] and majority of the cases are asymptomatic [5] but a small proportion presents with symptoms like epigastric pain and melena [2].
The characteristic histology of schwannoma is the presence of both hypercellular (Antoni A) and hypocellular (Antoni B) areas. But in GS, it is composed predominantly of Antoni A areas. The tumour exhibits sheets of spindle cells with vague nuclear palisading, peritumoural lymphoid cuff, variable myxoid stroma and no encapsulation. S100 immunoreactivity and CD117 immunonegativity confirms the diagnosis of GS [1,4].
The clinicians face a great challenge in diagnosing GS since it does not show any characteristic radiological features to distinguish it from other common mesenchymal tumours like GIST, the treatment and prognosis of these two tumours differ. GS are considered as benign tumours with a favourable prognosis and the treatment of choice is complete surgical resection if symptomatic [2,3].
In this study, the clinicopathological features including follow-up data of GS was reviewed and the benign nature of this tumour was confirmed. Although rare, GS needs to be considered as one of the differentials of gastric mesenchymal tumours. Since, there are only a few case reports published in Indian literature, hence, this study was undertaken to analyse the histopathological, immunohistochemical, radiological characteristics and follow-up data of GS.
Materials and Methods
The present study was a retrospective descriptive study conducted in the Department of General Pathology in a tertiary care hospital in Southern India from January 2010 to December 2019. All procedures performed in the current study were approved by Institutional Review Board (IRB) Minutes number: 13442, dated 23.09.2020.
Eight cases of histopathologically and immunohistochemically proven GS were retrieved from the archives of the Department of General Pathology. The clinical information along with tumour size and gross details were retrieved from the electronic database. Haematoxylin and Eosin (H&E) stained slides and immunohistochemical slides were analysed systematically and confirmed by two independent pathologists. Radiological features were reviewed on the institutional Picture Archiving and Communication System (PACS) by a Radiologist.
The histological features examined include the architecture of the tumour, mitotic count per 5 mm2 total area, peritumoural lymphoid reaction, presence of ulceration, blood vessel changes, nature of extracellular matrix and presence of tumour encapsulation. The immunohistochemical slides S100, SOX10, DOG1, CD117, Smooth Muscle Actin (SMA) and desmin were reviewed.
Statistical Analysis
Descriptive statistical analysis including frequency, percentage and mean of eight cases were done.
Results
There were eight patients with GS identified over the study period. The patient age ranged from 39 to 71 years (mean, 55 years) with male: female ratio being 1:3. The major presenting symptom was abdominal discomfort noted in 6 cases (75%). Out of these six cases, two had associated abdominal pain (33.3%) and two other patients also had early satiety (33.3%). Two out of 8 patients (25%) were asymptomatic and were detected incidentally during evaluation for unrelated medical issues [Table/Fig-1].
Clinicopathological summary of gastric schwannoma.
| Case | Age | Gender | Symptoms | Size(cm) | S100 | SOX10 | Follow-up duration |
|---|
| 1 | 69 | M | Incidental | 6 | + | nil | 9 years |
| 2 | 71 | F | Abdominal discomfortEarly satiety | 3 | + | nil | - |
| 3 | 42 | F | Abdominal discomfort | 4.5 | nil | + | 4 months |
| 4 | 39 | F | Abdominal discomfort | 4 | + | nil | - |
| 5 | 70 | F | Abdominal discomfort Early satiety | 7 | + | nil | 2 months |
| 6 | 58 | M | Abdominal discomfort Abdominal pain | 8 | + | + | - |
| 7 | 45 | F | Incidental | 5 | + | nil | - |
| 8 | 48 | F | Abdominal discomfort Abdominal pain | 4 | nil | + | 6 months |
Nil- Not done
In this study of eight cases, five patients underwent wedge resection (63%), two had sleeve gastrectomy (25%) and one had wide local excision (12%).
Gross Pathology
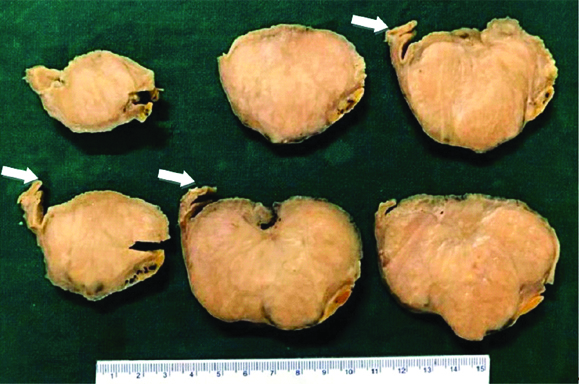
Six out of eight cases of GS involved the body of stomach (75%) which is considered as the most common site while the rest (two out of eight cases) were seen in the gastric fundus (25%). The tumour was located predominantly in the submucosal plane in four out of 8 cases (50%), subserosa in two out of 8 cases (25%) and submucosal and subserosal plane in two out of 8 cases (25%). Seven out of eight cases of GS had a nodular growth pattern (87.5%) while one case had an exophytic growth pattern (12.5%). The tumour size ranged from 3 to 8 cm (mean: 5.2 cm). All cases had a uniform grey white to yellow cut surface with well circumscribed borders and without a definite capsule [Table/Fig-2]. There was no ulceration of the overlying mucosa, necrosis or cystic areas in these tumours.
Well circumscribed grey white tumour arising from stomach wall (white arrow).
Histologic Features
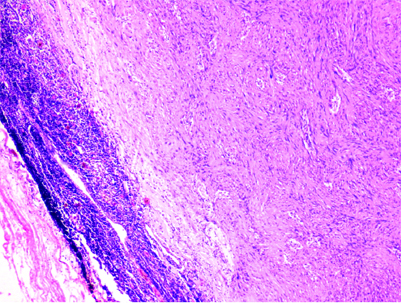
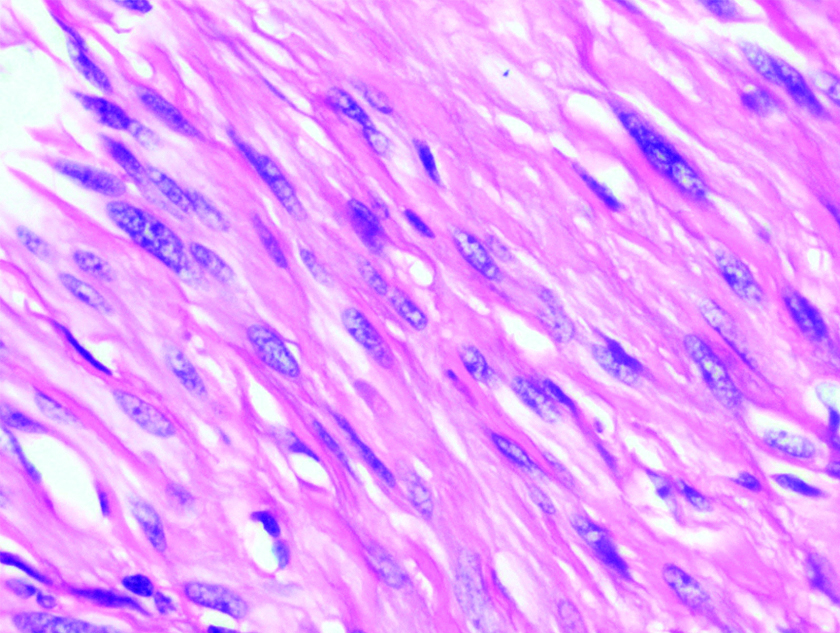
Microscopically, all GSs were characterised by fascicles of spindle cells exhibiting mild nuclear pleomorphism with evident peritumoural lymphoid cuff [Table/Fig-3]. Three out of eight cases (37.5%) showed vague nuclear palisading [Table/Fig-4] but none of them showed definite Antoni A and Antoni B areas. All the tumours had no definite capsule. Mitotic activity was not evident. The intervening stroma showed oedema (3/8), collagenisation (5/8) and calcification (1/8). None of these cases had cystic areas, necrosis, myxoid stroma or hyalinised vessels.
Subserosal tumour with peritumoural lymphoid cuff (H&E 40X).
Vague palisade of spindle cells with mild nuclear pleomorphism and dispersed chromatin (H&E 400X).
Immunohistochemical Findings
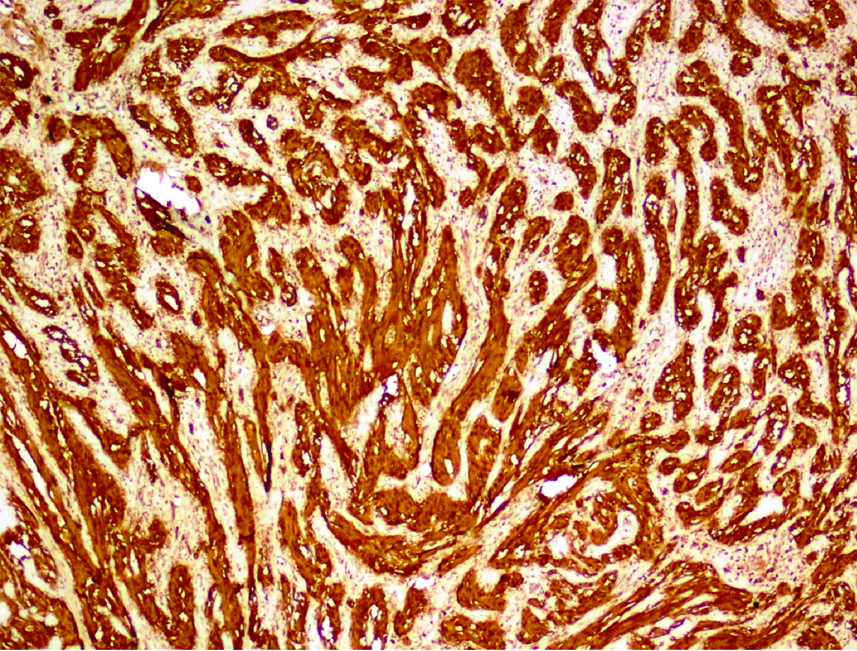
S100 immunohistochemical stain was available in six out of eight cases and all six cases showed strong positivity [Table/Fig-5]. SOX10 was performed only in three cases and all showed immunoreactivity. Among these eight cases, one had both S100 and SOX10 immunoreactivity. All eight cases were immunonegative for DOG1, CD117 and Smooth Muscle Actin (SMA).
Tumour cells with diffuse S100 protein staining (S100,40X).
Radiology
Seven cases had Computed Tomography (CT) while one had Positron Emission Tomography (PET). All lesions were well defined with homogenous attenuation and mild enhancement on imaging, one showed coarse calcific foci within [Table/Fig-6]. Four out of 8 (50%) of the lesions had an exophytic growth pattern, three out of 8 (37.5%) exhibited an endophytic growth pattern while one patient had a combination of these patterns. A differential diagnosis of GIST was considered in all these lesions.
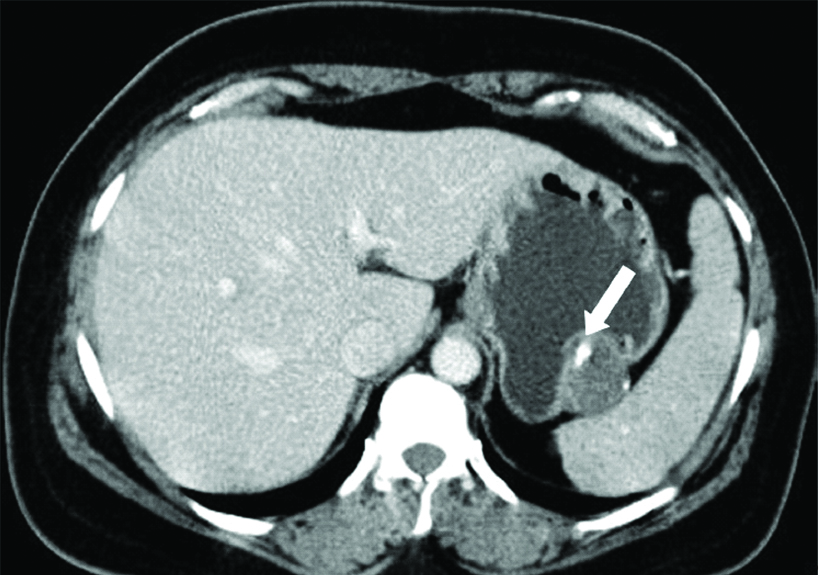
Contrast enhanced CT scan of a 45-year-old female with Gastric Schwannoma (GS) shows an exophytic hypo enhancing lesion from the fundus of the stomach with coarse calcification within (white arrow).
Other Preoperative Findings
Although all eight patients had undergone gastroscopy, only five patients had endoscopic mucosal biopsies. The remaining three patients had no significant mucosal abnormalities and hence, biopsy was not taken. There were no significant findings in the biopsies taken except for one which was reported as spindle cell neoplasm. Only one case had endoscopic ultrasound guided fine needle aspiration smears which was reported as benign spindle cell neoplasm.
Follow-up
The follow-up was available for only four patients ranging from two months to nine years and all were alive during this period. One patient was diagnosed with high grade neuroendocrine tumour in the liver, eight years after the diagnoses of GS. There was no evidence of recurrence in these patients.
Discussion
In general, gastrointestinal schwannomas originate from the nerve plexus of gut wall, unlike the conventional schwannomas, which develop from peripheral nerve anywhere in its course. The stomach is the most common location for these lesions in the entire GI tract and it accounts for 0.2% of all gastric tumours [1-3,9]. Only a few studies have been done on GS and most are published in western literature. The largest case series was done by Voltaggio L et al., which included 51 cases of GS in the year 2012 [4]. Since only few case reports were available in Indian literature, this retrospective study of eight cases of GS over a period of 10 years was undertaken.
In this study, GS was commonly seen in the fifth to eighth decade of life having a male to female ratio of 1:3 and the mean age at presentation was 55 years as described in the other studies published in the literature [1,2,9-13]. In this study, the most common symptom was abdominal discomfort in 6 out of 8 cases (75%) followed by abdominal pain and early satiety as opposed to being incidental in the previously published studies. A case of GS presenting as gastroduodenal intussusception has also been reported by Yang JH et al., recently in the literature [14]. In the present study, 75% of patients were symptomatic which possibly could be due to large tumour size.
On cross-sectional imaging, all the lesions were well defined with mild enhancement in keeping with the existing literature [15]. The pattern and degree of enhancement in the different phases of CT examination were not assessed as the CT protocol was not uniform in the series. Majority of the cases were homogenous in attenuation. Interestingly one case (1/8, 12.5%) had coarse calcific foci within. None of present study cases had cystic degeneration or necrosis. Majority of the lesions had an exophytic growth pattern (50%) followed by endophytic pattern (37.5%) and one patient (12.5%) had a mixed growth pattern with endophytic and exophytic components. Studies’ evaluating the imaging features of GS is sparse in the literature. Levy AD et al., in their study on gastrointestinal schwannomas showed 40% of their cases had exophytic or endophytic growth pattern while a mixed pattern of growth was seen in 20%. They did not report calcification in any of their cases [15]. In the study by He MY et al., which looked at CT features to differentiate gastrointestinal schwannoma and GIST, calcification was seen in 26.7% of schwannomas. Larger size (≥5 cm) and cystic change were found to have highest sensitivity and specificity for GIST among the various CT features studied [16]. GIST was considered a differential diagnosis in all cases in the present study on imaging. Though, lack of necrosis and homogenous attenuation has been suggested as findings in favour of Schwannoma over GIST, in practice these are non-specific, and imaging cannot often reliably differentiate between the two. GS need to be considered in the list of differential diagnosis in patients with above radiological features.
In this study, despite no obvious endoscopic findings, five cases underwent gastroscopic mucosal biopsy due to clinical suspicion of a neoplasm. One case out of five was reported as spindle cell neoplasm on endoscopic biopsy, the same patient also had an endoscopic ultrasound guided fine needle aspiration smears. Hence, it is inferred that routine gastroscopy is found to be less beneficial due to the deeper location of the tumour mainly in subserosal and submucosal plane. Endoscopic ultrasound guided fine needle aspiration biopsy may be superior to routine endoscopic biopsy with less sampling error [1,9].
A preoperative diagnosis of GS was found to be difficult and challenging and no specific clinical symptom or diagnostic modality was unique to this tumour, in this study. Therefore, histopathological examination and immunohistochemical markers still are considered as the gold standard for diagnosing GS. In this study, all cases of GS were composed of spindle cells usually arranged in fascicular pattern with peritumoural lymphoid cuff, often with germinal centres, which are considered as characteristic histological features of GS in the literature [1-4,9-13,17]. It has been described in literature that the tumour can show secondary changes like oedema, collagenisation, myxoid stroma, cystic degeneration, and calcification irrespective of the size of the tumour. In this study, majority of the tumours (75%) had one or the other secondary changes like stromal oedema, collagenisation, and calcification. GS differ from conventional schwannomas in many ways such as lack of encapsulation, verocay body formation, xanthoma cells, vascular hyalinisation, and the presence of peritumour lymphoid cuff [4,11,13]. These findings were confirmed in this study.
The main differential diagnosis of a gastric submucosal lesion is GIST and smooth muscle neoplasm. In this study, authors excluded GIST mainly by histology and DOG1 and CD117 immunonegativity. The possibility of smooth muscle neoplasm was also ruled out by non-immunoreactivity for SMA. In this study, six out of eight cases of GS were located in gastric body (75%) and in submucosal plane (75%), congruent with other studies [1,13].
Immunohistochemistry plays a vital role in differentiating various types of submucosal mesenchymal tumours. In present study, GS showed strong positivity for S100 protein and SOX10, and were immunonegative for DOG1, CD117 and smooth muscle markers, like all other studies [1-4,9-13,17,18].
In this study, all cases underwent surgical resection mainly due to the symptomatic nature of the tumour. Generally, the treatment of GS depends on the size and depth of the tumour. Surgical excision of GS is suggested for a tumour located within the muscularis propria or size >3 cm due to high risk of perforation [1,4,11,13]. None of the cases in this study showed any malignant transformation. Rarely, malignant GS have been reported in the literature and such patients should be followed-up for the next five years [1]. As observed in present study, GS has a good prognosis with no risk of recurrence or metastasis.
Limitation(s)
In this study, the number of cases were too small to conclude GS as one of the important differential diagnosis of gastric mesenchymal tumours.
Conclusion(s)
Schwannomas are considered as slow growing, rare and mostly asymptomatic gastrointestinal mesenchymal tumours, different from conventional schwannomas. Preoperative diagnosis of GS can be challenging as the clinical, endoscopic, and imaging findings are not specific. GS should be considered as one of the differential diagnosis if the tumour is submucosal in location with homogenous attenuation, and lack of necrosis on imaging studies. The histopathological features including immunohistochemical examination, is the gold standard to diagnose GS. Complete surgical excision is the treatment of choice for GS, it is usually benign and the prognosis is good.
Nil- Not done